-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2014; 4(2): 30-34
doi:10.5923/j.pc.20140402.02
Crystal Structure of Mongolian Phosphorite Minerals and Mechanochemistry
P. Jargalbat1, G. Batdemberel2, Sh. Chadraabal2, D. Sangaa3, J. Amgalan4
1School of Pharmacy and Bio-Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
2School of Applied Science, Mongolian University of Science and Technology, Ulaanbaatar, Mongolia
3Institute of Physics and Technology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia
4Institute of Chemistry and Chemical Technology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia
Correspondence to: P. Jargalbat, School of Pharmacy and Bio-Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
We have carried out crystal structure investigations of natural and mechanochemically activated samples of Burenkhan, Tsakhir uul and Aldarkhan phosphorite deposits (The North Mongolia) by x-ray, neutron and synchrotron powder diffraction techniquies. We have determined the exact mineral contents of the phosphorite ore and crystallochemical formula of each mineral. It was established that natural samples of Tsakhir uul and Aldarkhan phosphorite deposits contain flourite (CaF2) among the main ore minerals ((Ca)5(PO4)3F and SiO2). The amount of CaF2, in the sample of Tsakhir uul is more than in the Aldarkhan sample. It was shown that as a result of mechanochemical activations any new crystal phases formed. Strong distortion in the crystal lattice of flourapatite, especially, in the PO4-tetrahedrons that possibly lead to increasing of the lattice energy and more solubility of apatite after mechanochemical activation.
Keywords: Mongolian phosphorite, Crystal structure, Mechanochemistry, X-ray diffraction, Rietveld method
Cite this paper: P. Jargalbat, G. Batdemberel, Sh. Chadraabal, D. Sangaa, J. Amgalan, Crystal Structure of Mongolian Phosphorite Minerals and Mechanochemistry, Physical Chemistry, Vol. 4 No. 2, 2014, pp. 30-34. doi: 10.5923/j.pc.20140402.02.
1. Introduction
- According to the survey of A.I.Ilyin and B.Z. Blyskovskii (1984), the Khuvsgul phosphorite basin spans from the northest Ukhaa river deposit with square of 30000 km2 to southern Burenkhan deposit along a longitude of 300 km and covers more than 50 deposits, ranking at the first place in Asia by large resources of high-quality phosphate of 4.5 billion tonnes [1].As geological structure, the Aldarkhan phosphorite deposit is rather simply located on the territory of Zavkhan aimag. Today it is estimated that this deposit has ore of 465 thousand tonnes. The Tsakhir uul phosphorite deposit is located in Yaruu sum, Zavkhan aimag and covers the north-western hills of Tsakhir uul, which is at 12-20 km north-westward from the Alagiin davaa deposit [1].The phosphorite mainly occurs in the lower parts of carbonate layer of Vendian-Cambrian age. Its lithological and paleogeographic features are the same for all deposits. Our objectives in this work are to study the crystal structure of natural and mechanochemically activated samples of phosphorite deposits in Tsakhir uul, Aldarkhan of Zavkhan aimag, Burenkhan of Khuvsgul aimag of Mongolia by x-ray, neutron and synchrotron powder diffraction techniques and to summarise the results.
2. Experiment and Results
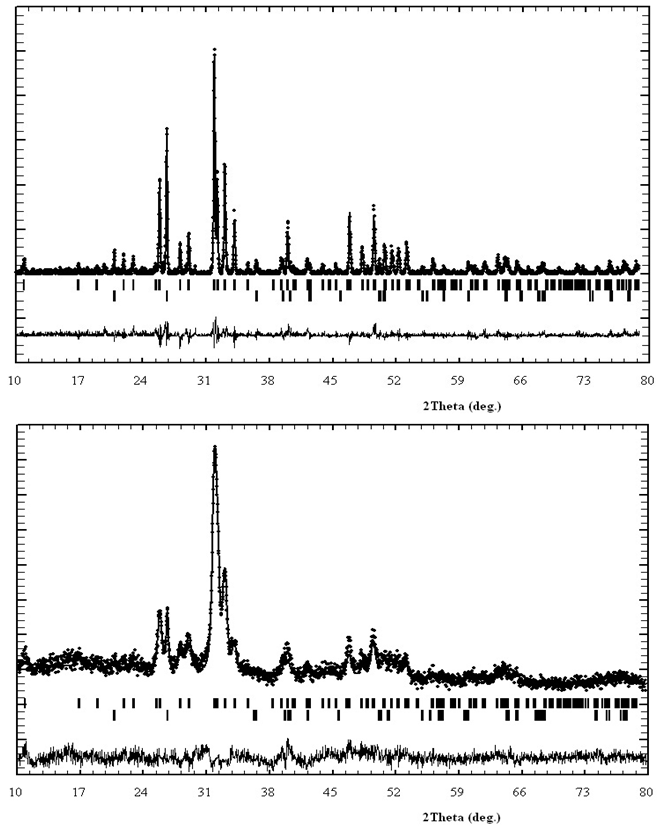
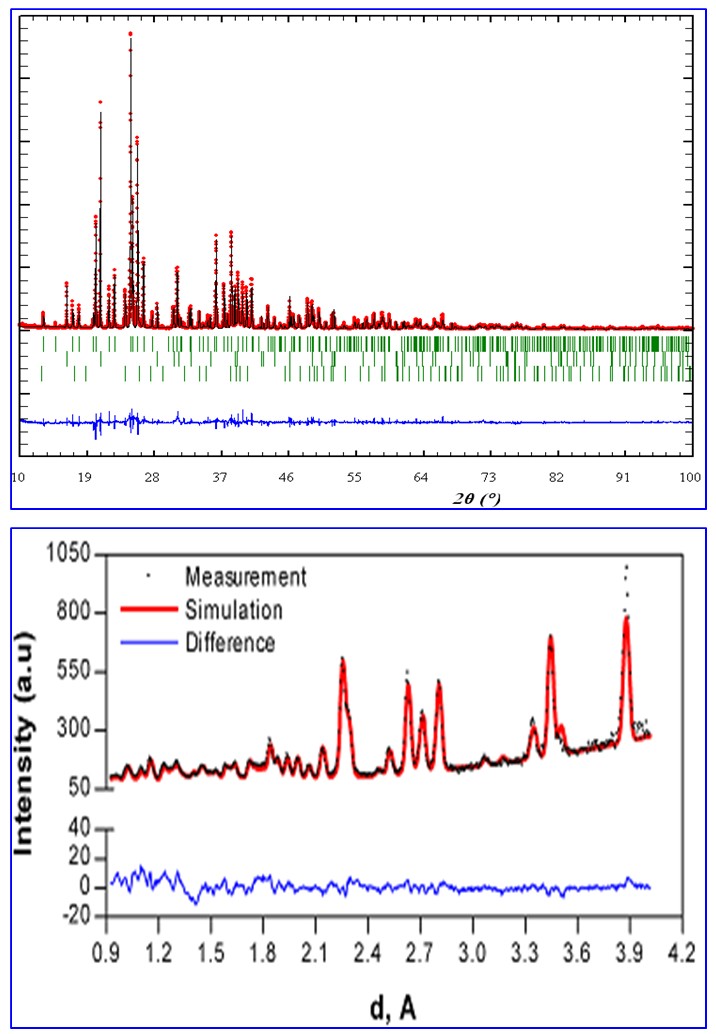
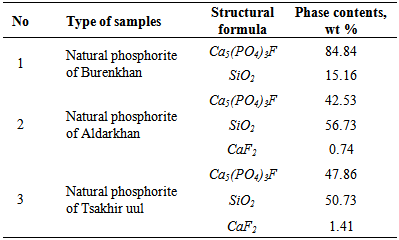
- The phosphate of Burenkhan phosphorite deposit, Khuvsgul, Mongolia, has been studied by X-ray diffraction and the mechanochemical activation’s effect in the structural parameters of fluorapatite has been determined (figures 1, 3, 4 and table 1) [1, 2].In the result of comparative studies in dependence crystalline structures of natural minerals of phosphorite in Zavkhan and Khuvsgul aimags by X-ray diffraction method, it is proved that the natural samples of Tsakhir uul and Aldarkhan, Zavkhan, consist of the phases of fluorapatite (Ca)5(PO4)3F, quartz SiO2 and the fluorite (CaF2). The sample of Tsakhir uul, Zavkhan, contains relatively more fluorite phase (CaF2) than the sample of Aldarkhan (table 2) [3].In addition, the position sorts, chemical bonds and electronic density of divalent cations (Sr2+, Ba2+) in the flourapatite of activated and unactivated samples from the Burenkhan phosphorite deposit, Khuvsgul, have been studied depending on mechanochemical process by X-ray diffractometer and fourier synthesis methods. And it is explained that the electronic density growth in the anion’s position has more ionic characteristic than their forming bonds; that F↔OH occupation occurs in the same time of the formation of hydrogen bonds, so this destabilizes the crystal lattice of apatite. Furthermore, it may be a reason that its solubility is increased as the result of mechanochemical activation [4, 5].The studies of crystal structure of natural phosphorite from Burenkhan, Mongolia, by synchrotron powder diffraction (SR) method show that the phosphorite ore consists of three minerals: hydroxylflourapatite Ca10(PO4)6(F, OH), quartz (α-SiO2) and dolomite Ca0.43Mg(CO3)2 [5, 6]. For hydroxylflourapatite and dolomite, the calcium content is strongly deviated from stoichiometry. In the refinement of diffraction spectrum, the composition of minerals contained in the samples of phosphorite is determined and the exact formula can be written:
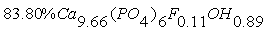
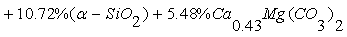 The OH content in crystal structure of phosphorite of Burenkhan, Khuvsgul, Mongolia, is determined by the TOF-neutron diffraction method, and the chemical formulas of each sample are:
The OH content in crystal structure of phosphorite of Burenkhan, Khuvsgul, Mongolia, is determined by the TOF-neutron diffraction method, and the chemical formulas of each sample are: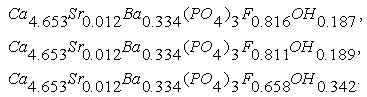 The results of this research work are briefly shown as integrated form in figures 1, 2, 3 and 4 and tables 1 and 2 [2-6].
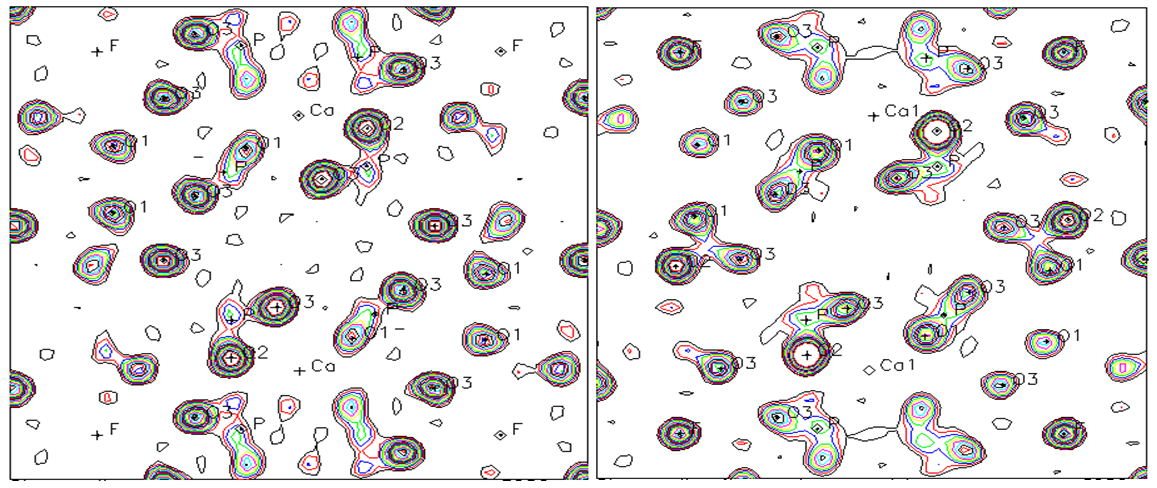
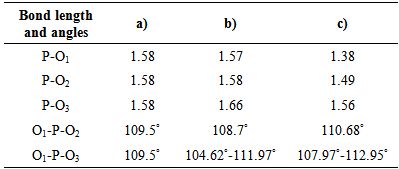
The results of this research work are briefly shown as integrated form in figures 1, 2, 3 and 4 and tables 1 and 2 [2-6].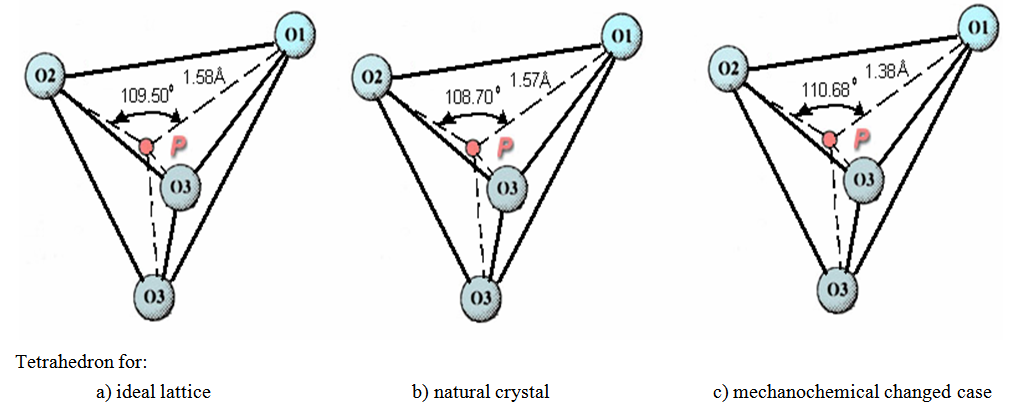 | Figure 4. Change of bond length and angles of PO4 -tetrahedron in the apatite crystal lattice |
|
|
3. Conclusions
- In the result of X-ray diffraction research, the formula of mineral structure of phosphorite (Burenkhan) is written as follows:
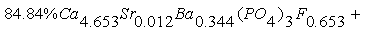
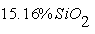 In phosporites of Burenkhan, Aldarkhan and Tsakhir uul deposits the main phosphorite mineral is flourapatite (Ca)5(PO4)3F with the space group P63/m. The mineral compositions of phosphorite can be expressed for the three deposits as the next:
In phosporites of Burenkhan, Aldarkhan and Tsakhir uul deposits the main phosphorite mineral is flourapatite (Ca)5(PO4)3F with the space group P63/m. The mineral compositions of phosphorite can be expressed for the three deposits as the next: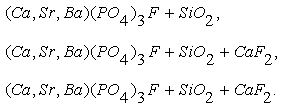 As the result of TOF-neutron diffraction technique, the coordinate and component of phosphorite anion (OH) of Burenkhan are determined:
As the result of TOF-neutron diffraction technique, the coordinate and component of phosphorite anion (OH) of Burenkhan are determined: 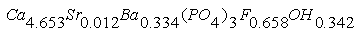 In the result of synchrotron radiation diffraction, the formula of the structure of phosphorite mineral of Burenkhaan is as follows:
In the result of synchrotron radiation diffraction, the formula of the structure of phosphorite mineral of Burenkhaan is as follows: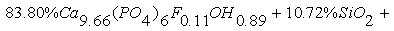
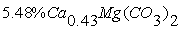 It is explained that F↔OH occupation occurs in same time of formation of hydrogen bonds, so this destabilizes the crystal lattice of apatite, furthermore, it may be a reason that its solubility has increased as the result of mechanochemical activation. The mechanochemical activation hadn’t any result the observation of any new crystalline phases. However, that the amorphisation occurs in crystal lattice of the apatite, for example, by observation of amorphisation of PO4 tetrahedron the energy of crystal lattice has increased and it could lead to be increased the solubility of the apatite.
It is explained that F↔OH occupation occurs in same time of formation of hydrogen bonds, so this destabilizes the crystal lattice of apatite, furthermore, it may be a reason that its solubility has increased as the result of mechanochemical activation. The mechanochemical activation hadn’t any result the observation of any new crystalline phases. However, that the amorphisation occurs in crystal lattice of the apatite, for example, by observation of amorphisation of PO4 tetrahedron the energy of crystal lattice has increased and it could lead to be increased the solubility of the apatite.  Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML