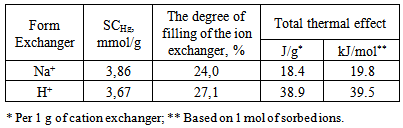-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2014; 4(1): 16-19
doi:10.5923/j.pc.20140401.03
Thermokinetics of Mercury (II) Cations’ Sorption on the Carboxycationite
Elena Zauer
Volgograd State Technical University
Correspondence to: Elena Zauer, Volgograd State Technical University.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Thermokinetics of sorption of mercury (II) acetate solution on the carboxylic acid cationite KB-4 in the sodium and hydrogen forms has been investigated by microcalorimetric method. The influence of the concentration of the sorbed ion in solution on sorbtion thermokinetics has been investigated. Total heat of sorption has been determined.
Keywords: Microcalorimetry, Sorption, Mercury, Carboxylic acid cationite-4 KB
Cite this paper: Elena Zauer, Thermokinetics of Mercury (II) Cations’ Sorption on the Carboxycationite, Physical Chemistry, Vol. 4 No. 1, 2014, pp. 16-19. doi: 10.5923/j.pc.20140401.03.
1. Introduction
- Ion exchange processes are widely used in industry, in protection of the environment and in analytical chemistry due to their high selectivity, low energy cost and relative ease of ion exchange process.The most commonly materials used for the ion exchange are ion exchange resins having a different nature and structure of the polymer matrix (including styrene) and containing various ionic groups capable of exchanging cations and anions from solutions. Efficient use of ion exchange material requires accurate data on the kinetics and mechanism of interaction of compounds extracted from an ion exchanger. Investigations of these issues were presented in a number of monographs and reviews [1-5]. To investigate the patterns and features of ion exchange processes different methods namely potentiometric titration, IR spectroscopy, atomic absorption spectrometry and X-ray fluorescence are used, the most informative one proved to be calorimetry. The first calorimetric studies of ion exchange processes were performed by the authors of [6, 7], which gave impetus to the widespread use of calorimetry for the study of sorption processes. At present, the thermal effects of sorption allows to study the influence of the type of an ion exchanger, its ionic form, nature and the amount of bridge forming fragment, the nature of the sorbed ions and co-ions on the kinetics and mechanism of the process.A number of studies are known in which calorimetry method was used for investigation of the kinetics and mechanism of sorption of copper, nickel, cobalt, manganese and zinc on carboxylic cation exchangers type CB-2, CB-2e, CB-4, the KMT [8-13]. Continuing microcalorimetric studies initiated in [13, 14], in the presented work the features of the sorption of mercury (II) acetate solutions of the carboxyl cation exchanger KB-4 in the hydrogen and sodium forms has been investigated.
2. Experimental
- Cationite KB-4 in salt (Na) and hydrogen forms was prepared according to [15]. A solution of mercury (II) was prepared from its acetate and has been standardized by the method of acidimetric titration [16]. Determination of SEHg sorption capacity (mmol g-1) of the both forms of an ion exchanger was performed according to [2, pp. 114] as follows. A sample of cationite was mixed with mercuric acetate solution, after 3 days mercury (II) concentration has been determined by acidimetric titration. Calculation of the sorption capacity was carried out according to the formula
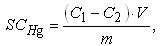 where C1 and C2 are concentrations (mol L-1) of mercury in solution before and after sorption; V is the volume of solution (L); m is mass of the cationite sample (g).Sorption kinetics was studied by microcalorimetric method [17] on the differential automatic calorimeter DAK-1-2M at 30°C. Weighed sample of 0.139 g of dry cation exchanger KB-4 was placed in a glass vial. An aliquot (0.35 mL), 0.5N solution of mercuric acetate (II) was poured into a glass vial with a bottom in the form of a thin-walled balloon. With the help of a special device, both vials were introduced into the calorimeter cell and thermostated. At the end of thermostating a thin-walled vial has been broken and thermokinetics curve of the sorption process has been registrated by electronic recorder.Effect of the mercury concentration in solution on sorption kinetics were examined on C80 Setaram calorimeter at 30℃. Sodium form of cationite exchanger has been used.The degree of filling of the ion exchanger by ion absorbed (%) has been calculated by the formula
where C1 and C2 are concentrations (mol L-1) of mercury in solution before and after sorption; V is the volume of solution (L); m is mass of the cationite sample (g).Sorption kinetics was studied by microcalorimetric method [17] on the differential automatic calorimeter DAK-1-2M at 30°C. Weighed sample of 0.139 g of dry cation exchanger KB-4 was placed in a glass vial. An aliquot (0.35 mL), 0.5N solution of mercuric acetate (II) was poured into a glass vial with a bottom in the form of a thin-walled balloon. With the help of a special device, both vials were introduced into the calorimeter cell and thermostated. At the end of thermostating a thin-walled vial has been broken and thermokinetics curve of the sorption process has been registrated by electronic recorder.Effect of the mercury concentration in solution on sorption kinetics were examined on C80 Setaram calorimeter at 30℃. Sodium form of cationite exchanger has been used.The degree of filling of the ion exchanger by ion absorbed (%) has been calculated by the formula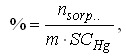 where nsorp. is the amount of absorbed mercury (in mmol). Quantity of mercury sorbed was determined as the difference between its concentration in the solution before and after calorimetry has been completed, using acidimetric titration method.
where nsorp. is the amount of absorbed mercury (in mmol). Quantity of mercury sorbed was determined as the difference between its concentration in the solution before and after calorimetry has been completed, using acidimetric titration method.3. Results and Discussion
- Cationite KB-4 under investigation refers to complex forming ionites on which the sorption of transition metal ions is due to the simultaneous formation of an ionic bond with an oxygen atom of hydroxyl group and coordination to carbonyl oxygen [2]. Furthermore, the authors of [18] on studying the mechanism of sorption of mercury on the carboxyl cationite SG-1 by the method of potentiometric titration have found that mercury, regardless of its concentration in the solution is characterized by the formation of complexes with two carboxyl groups of the ionite
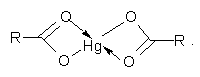 According to [19] the formation of complexes of this composition occurs with maximum evolution of heat. It should also be noted that the formation of structures similar to those under consideration, requires energy loss associated with deformation of the polymer network [8].The Figure 1 shows the curves of thermokinetics mercury sorption on weakly acidic cation exchanger KB-4 in the salt (Na+) (curve 1) and hydrogen (curve 2) forms. All sorption processes studied are exothermic. The nature of the curves is similar as on 2 or 3 min. after start a decrease in the sorption heat flux intensity has been observed. The initial increase of the intensity of heat evolution can be explain by the fact that sorption mainly occurs due to the functional groups located on the surface of the ion exchanger pellet. The subsequent decrease of the intensity of heat evolution may be due to the necessity of deep diffusion of sorbed ions into pellets of cationite associated with certain energy loss [8, 9].
According to [19] the formation of complexes of this composition occurs with maximum evolution of heat. It should also be noted that the formation of structures similar to those under consideration, requires energy loss associated with deformation of the polymer network [8].The Figure 1 shows the curves of thermokinetics mercury sorption on weakly acidic cation exchanger KB-4 in the salt (Na+) (curve 1) and hydrogen (curve 2) forms. All sorption processes studied are exothermic. The nature of the curves is similar as on 2 or 3 min. after start a decrease in the sorption heat flux intensity has been observed. The initial increase of the intensity of heat evolution can be explain by the fact that sorption mainly occurs due to the functional groups located on the surface of the ion exchanger pellet. The subsequent decrease of the intensity of heat evolution may be due to the necessity of deep diffusion of sorbed ions into pellets of cationite associated with certain energy loss [8, 9].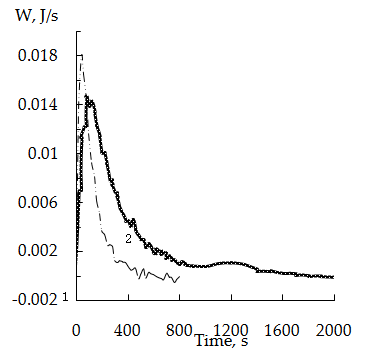 | Figure 1. Thermokinetics sorption curves of mercury (II) on sodium (1) and hydrogen (2) forms the cation exchanger KB-4 |
|
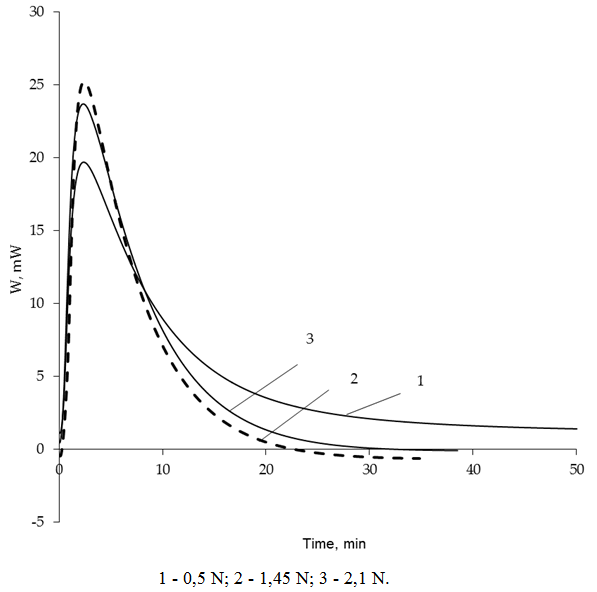 | Figure 2. Thermokinetic sorption curves of Hg from solutions of different concentrations |
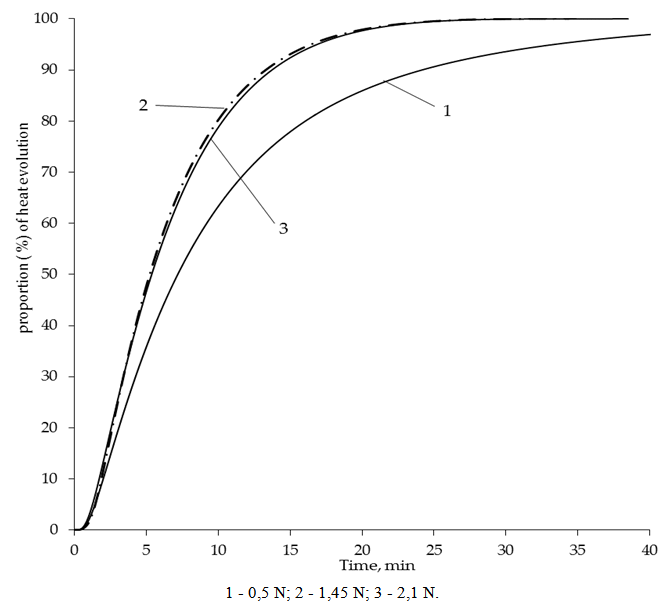 | Figure 3. Dependence of heat releasing on the mercury concentration in solution |
4. Conclusions
- Thus thermokinetics of sorption of mercury (II) acetate solution on a hydrogen and a sodium cation forms of carboxyl cationite has been investigated by microcalorimetric method. It was shown that at close values of sorption capacity and the degree of filling of the ion exchanger heat evolution on the H-form is twice as much as that in the case of the Na-form. The influence of the concentration of metal sorbed on the rate and duration of sorption has been found.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML