-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2014; 4(1): 11-15
doi:10.5923/j.pc.20140401.02
Thermokinetics of Sorption of Mercury (II) Iodide Complexes on Sulphocationite Ku-2-8 Modified by Crystal Violet
Zauer E. A.
Volgograd State Technical University, Volgograd, 400131, Russian Federation
Correspondence to: Zauer E. A. , Volgograd State Technical University, Volgograd, 400131, Russian Federation.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Thermokinetics of iodide complexes of mercury (II) sorption on sulphocationite KU-2-8 modified by basic dye crystal violet has been studied.
Keywords: Calorimetry, Sorption, Crystal violet, Mercury ions (II), Ion associates
Cite this paper: Zauer E. A. , Thermokinetics of Sorption of Mercury (II) Iodide Complexes on Sulphocationite Ku-2-8 Modified by Crystal Violet, Physical Chemistry, Vol. 4 No. 1, 2014, pp. 11-15. doi: 10.5923/j.pc.20140401.02.
1. Introduction
- High selectivity, low energy cost and relative ease of ion exchange process promotes its wide use in industry, environmental and analytical chemistry.The most commonly used material for the realization of ion exchange processes are the ion exchange resins. Their selectivity is variable. Strongly acidic and strongly basic ion exchangers, for example, are characterized by low selectivity. To improve it synthetic incorporation of chemically bound functional groups into a polymer molecule as well as sorption modification of ion exchange resins by means of organic reagents are used. Dyes are one the most commonly used reagents for modifying sorbents. There are many publications in the literature in which ion exchangers have been used for modifying of dyes.Thus, for the determination of Cd (II), Co (II), Cu (II), Ni (II), Zn (II), Fe (III) and Pb (II) ions in water, the authors [1,2] used modified xylenol orange Amberlite XAD-7 and XAD-2 for pre-sorption concentration and subsequent analysis by flame atomic absorption spectrophotometry. In [3] and [4] conducted modification Amberlite XAD-2 and Amberlyst A-26 Pyrocatechol purple has been used. The authors [5] have used XAD-2 alizarin red S for Amberlite XAD-2 modification for lead (II), cadmium (II), zinc (II) and nickel (II) analysis.The content of heavy metals in food samples has been determined by solid-phase spectrophotometry, using preliminary sorbed lead iodide complexes on sulphocationite KU-2, modified by basic blue [6], wheras zirconium sorption has been performed on AB-17 modified by eriohromium black T [7]. In [8] rhodamine Zh was used as a modifier of cation exchanger KU-2 for the simultaneous separation of ionic mercury (II), iron (III) and zinc in the form of thiocyanate complexes and subsequent determination of their content in the sorbent phase by X-ray method.In the course of the sorption-spectroscopic and test analysis the authors [9-12] have successfully used polyurethanes pre-soaked in various dyes (eg, acridine yellow [11] as a sorbent as well as different sulfophtaleine dyes [12]), and then have extracted metal ions from solutions asin the form of ion associates with the dye.Efficient use of ion-exchange materials requires accurate data on the kinetics and mechanism of interaction of compounds extracted from an ion exchanger. For these purposes different methods including calorimetry are used the latter providing the most informative study of sorption processes.Calorimetry method was successfully used to study kinetics and mechanism of adsorption of simple ions by various industrial ion exchangers [13-17]. There are relatively fewer papers in which calorimetry is used to study sorbtion kinetics of complex ions and of modified conventional ion exchangers, even though preliminary binding of metal ions in the complexes, as well as the modification of ion-exchange resins, including surface, very often is used to enhance the selectivity of sorption processes. Among these works [18] and [19] may be noted in which ampholyte ANKB-35 has been investigated by microcalorimetric method. Paper [18] presents the results of thermochemical studies of competitive complexation in the system nickel ion (II)-amino acid – exchanger whereas in [19] the enthalpy of interaction of amino acid complexes of copper (II) has been investigated.In paper [20] calorimetry has been used for studying of rare metal sorbtion on phosphoracidic cationite KFP-12 from solutions incorporating fluoride and chlorine anions as well as their mixture.In this paper we tried to investigate some pecularities of energetics of sorbtion of iodide complexes of mercury (II) on highly acidic cation exchanger KU-2 by microcalorimetric method. Its surface has been modified by basic dye of the triphenylmethane series crystal violet (CV). It is known [21] that basic dyes such as crystal violet, (rhodamine X, rhodamine B, methylene blue, methyl violet, etc.) may form associates with halide ion and thiocyanate complexes of certain metals that are insoluble in water but soluble in the organic phase (for example, toluene). This property of dyes are widely used in analytical chemistry for the extraction-photometric determination of a number of elements, and mercury in particular [22].Selection of crystal violet in the ion exchange resin as a modifier is due to its high selectivity for mercury determination. This property of crystal violet is the basis of a well-known technique of extraction-photometric determination of mercury (II) in natural and waste waters [23]. This method has been selected as basic for the choice and composition of the complex ion.In this paper on adding of cationite KU-2 modified by crystal violet dye to a solution containing iodide anion complexes the latter are electrostatically attracted to them forming ionic associates in the sorbent phase. The mechanism of interaction of complex anions with a modified sorbent can be represented as follows:RSO3ˉCV+ + [HgI3]ˉ → RSO3ˉCV+−{[HgI3]-};2 RSO3ˉCV+ + [HgI4]2- → (RSO3ˉ CV+)2−{[HgI4]2-}.
2. Experimental
- To produce a modified original cation exchanger KU-2, prepared according to [24], a sample allowed to stand for 24 hours in 0,2% aqueous crystal violet solution and at the end of this time was collected by filtration, washed with distilled water and dried.A solution of mercury (II) was prepared from its acetate and was standardized by acidimetric titration [25].Mercury iodide complex was prepared immediately before conducting calorimetric studies by mixing of a solution of mercuric acetate (II) with an aqueous solution of potassium iodide. We’ve based on data from [23] that the increase of iodide concentration above 0,02 M predominantly leads to the formation of [HgI4]2-.Thermokinetics of sorbrion was studied by microcalorimetric method [26] on the differential automatic calorimeter DAK-1-2M at 30°C. Weighed sample (0,139 g or 0,278g) of dry modified cation KU-2 was placed in a thick-walled glass ampoule. An aliquot (0,35 mL) solution containing a complex of mercury iodide (II) was poured into a glass vial with a bottom in the form of a thin-walled balloon. With the help of a special device, both vials were introduced into the calorimeter cell and incubated. At the end of incubation, and a thin-walled vial was broken, thermokinetics curve of the sorption process was fixed by the recorder.
3. Results and Discussion
- Figure 1 - 4 presented thermokinetics sorption curves of mercury iodide complexes on the cation exchanger KU-2- 8 modified by crystal violet. All thermokinetics curves have the same character as sorption is accompanied by evolution of heat. On the initial portion one can see rapid heat evolution with maximum in 150 – 180 sec. Presence of a maximum can be explained by the occurrence of energetically more favorable sorption of mercury iodide complexes with a modifier on the surface of the sorbent grain. The subsequent decrease in the intensity of the heat flux can be caused by energy consumption penetration (diffusion) of adsorbed ions deep into the grain.
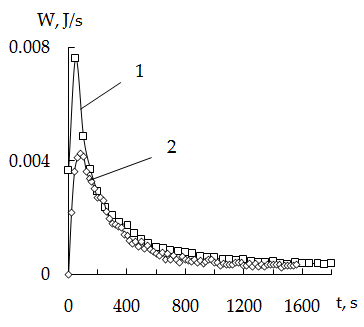
 | Figure 1. Thermokinetics sorption curves of mercury on KU-2 + CV. Dependence on the concentration of mercury 30оC (msorb. 0,139g); Hg concentrations 1-0,11 N; 2 - 0,22 N; 3-0,39 N |
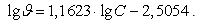 According to this equation logarithm of the rate constant of the iodine-mercury complexes sorption on cationite KU-2 modified by crystal violet is equal to -2,5747 corresponding to a rate constant about 2,66•10-3 s-1. The reaction order on mercury equal to a tangent of angle inclination of the dependence obtained is 1,1441, that is approaching to the first one.Variation of temperature leads to significant changes in sorption energetics (Figure 2) viz. temperature increase from 30°C to 50°C leads to decreases heat evolution. The total thermal effect decreases by almost an order of magnitude. (from 5,6 J to 0,66 J).
According to this equation logarithm of the rate constant of the iodine-mercury complexes sorption on cationite KU-2 modified by crystal violet is equal to -2,5747 corresponding to a rate constant about 2,66•10-3 s-1. The reaction order on mercury equal to a tangent of angle inclination of the dependence obtained is 1,1441, that is approaching to the first one.Variation of temperature leads to significant changes in sorption energetics (Figure 2) viz. temperature increase from 30°C to 50°C leads to decreases heat evolution. The total thermal effect decreases by almost an order of magnitude. (from 5,6 J to 0,66 J).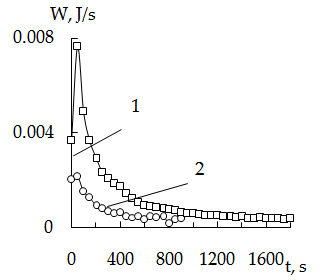 | Figure 2. Thermokinetics curves of mercury sorption on KU-2 + CV. Dependence on temperature msorb. = 0.139 g; Hg concentration -0,22 N; 1 - 50℃, 2 - 30℃ |
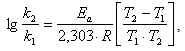 presented energy of activation of sorption process has been calculated equal to 16,6 kJ/mol. Where k1 and k2 are rate constants measured at two temperatures of T1 and T2 respectively; R-universal gas constant equal to 8,314 J/(mol·K).The pre-exponential factor A can be found from the equation
presented energy of activation of sorption process has been calculated equal to 16,6 kJ/mol. Where k1 and k2 are rate constants measured at two temperatures of T1 and T2 respectively; R-universal gas constant equal to 8,314 J/(mol·K).The pre-exponential factor A can be found from the equation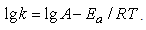 Value k at 30°C insertion in the equation gives a value A equal to 0,396.Taking into account that energy of activation of diffusiion processes changes in the range of 10-40 kJ/mol and that in accord with [28] external diffusion kinetics is realized at energies of activation smaller than 20 kJ/mol wheras internal diffusion occurs at energies of activation larger than 20-40 kJ/mol, one may come to the conclusion on predominace of external diffusion on the rate of sorbtion process.When the mass of cationite is increased twice (Figure 3) the intensity of heat evolution is also increased twice as much wheras the amount of heat evolved per 1 g of sorbent remains virtually unchanged an is equal to 17,1 J. Duration of heat evolution depends on the mass of the sorbent sample. In the case of 0,139g weight heat evolution process is completed within 800-1000 sec wheras for the mass of 0,278 g it’s more longer, viz.1200-1600 sec.
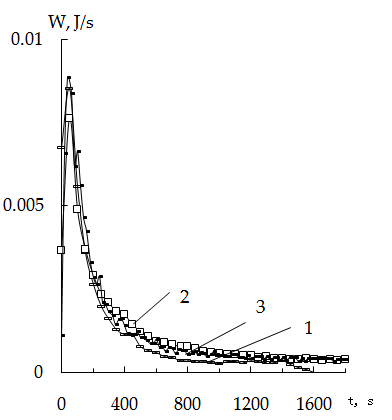
Value k at 30°C insertion in the equation gives a value A equal to 0,396.Taking into account that energy of activation of diffusiion processes changes in the range of 10-40 kJ/mol and that in accord with [28] external diffusion kinetics is realized at energies of activation smaller than 20 kJ/mol wheras internal diffusion occurs at energies of activation larger than 20-40 kJ/mol, one may come to the conclusion on predominace of external diffusion on the rate of sorbtion process.When the mass of cationite is increased twice (Figure 3) the intensity of heat evolution is also increased twice as much wheras the amount of heat evolved per 1 g of sorbent remains virtually unchanged an is equal to 17,1 J. Duration of heat evolution depends on the mass of the sorbent sample. In the case of 0,139g weight heat evolution process is completed within 800-1000 sec wheras for the mass of 0,278 g it’s more longer, viz.1200-1600 sec.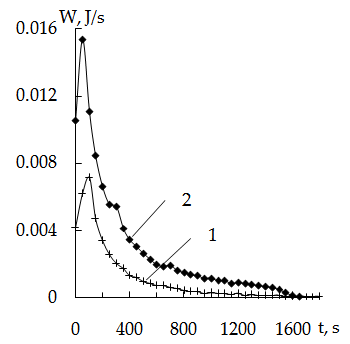 | Figure 3. Thermokinetics sorption curves of mercury increase on KU-2 + CV. Dependence on the mass of sorbent 30°C, Hg concentration - 0,22 N; msorb. 1-0,139 g, 2-0,278g |
4. Conclusions
- Thus in this study energetics of extraction process of iodide complexes of mercury ions from solution on cation exchanger KU-2 modified by crystal violet has been in vestigated. It was found that the order of sorption process is close to the first one. The rate constant is equal to 2,66·10-3 s-1. The value of energy of activation is equal to 16,6 kJ/mol suggesting predominace of sorption external diffusion mechanism. The magnitude of pre-exponential factor is equal to 0,261.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML