-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2013; 3(2): 39-47
doi:10.5923/j.pc.20130302.02
A Comprehensive Mechanism for Aromatic Nucleophilic Substitution in Aprotic Solvents: Derivation of the Whole Reaction Scheme for Third Order in Amine Kinetic Law
Cecilia E. Silvana Alvaro1, Norma S. Nudelman2
1Departamento de Química, Facultad de Ingeniería, Universidad Nacional del Comahue, Buenos Aires 1400, Neuquén, 8300, Argentina
2Departamento de Química Orgánica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Pab, II, P 3 Ciudad Universitaria, 1428, Buenos Aires
Correspondence to: Norma S. Nudelman, Departamento de Química Orgánica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Pab, II, P 3 Ciudad Universitaria, 1428, Buenos Aires.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
When aromatic nucleophilic substitutions (ANS) with amines are carried out in solvents of low permittivity, a third order in amine kinetic law is often determined. Initially considered an “atypical” behavior, this and other peculiar findings could be consistently accommodated in a new mechanism for ANS that we called the “dimer nucleophile” mechanism. At present, there are abundant studies in the current literature where the “dimer” mechanism has been observed. Though most of the reported 4th order ANS occurred with poor nucleophiles, we recently designed special systems that allowed the “dimer” mechanism be observed also with substrates where the first step is rate determining. In the present paper, the kinetic behavior and reactivity for ANS reactions performed with halonitrobenzenes and several bi- and poly-functionalized amines in toluene is reported. Special systems implying nucleophiles of flexible structure and appropriate rigid molecular geometry, regarding their hydrogen bond self-aggregation states were designed; the presence of non-nucleophilic hydrogen bond acceptor additives was also studied. It is shown that the nature of non-covalent weak interactions developed in aprotic media alter the properties of the nucleophile. Our results confirm that the association of amines in aprotic solvents plays an important role in defining the ANS mechanisms. An overall reaction Scheme is presented herewith, including determination of the partial k’s of the individual steps in the complex reaction.
Keywords: Aromatic Nucleophilic Substitution, Aprotic Solvents, Aggregation Effects, Overall Kinetic Treatments, “Dimer” Nucleophile Mechanism
Cite this paper: Cecilia E. Silvana Alvaro, Norma S. Nudelman, A Comprehensive Mechanism for Aromatic Nucleophilic Substitution in Aprotic Solvents: Derivation of the Whole Reaction Scheme for Third Order in Amine Kinetic Law, Physical Chemistry, Vol. 3 No. 2, 2013, pp. 39-47. doi: 10.5923/j.pc.20130302.02.
Article Outline
1. Introduction
- The elucidation of mechanisms of reaction is a main area of interest, not only because of its fundamental relevance, but also for projecting new practical routes in other fields of scientific and technological research. In this sense, an active investigation on Aromatic Nucleophilic Substitution (ANS) which includes fundamental[1-6] and applied[7-11] research is being developed at present. The interest stems from the fact that ANS can proceed through a variety of pathways[12, 13] and the many-sided nature of the catalysed ANS mechanisms[4]. Synthesis of polymeric materials[7], dyes[8], pharmaceuticals[9, 10] and other bioactiveagents[11] based on ANS have been recently reported, including a patented treatment of halohydrocarbons and polychlorinated biphenyls for the decontamination of groundwater[14].Though ANS by amines has been recognised to be extremely affected by the solvent[12, 15], only few attempts have been made to study the effects of solvent in a systematic way[16-20]. If ANS with amines and substrates activated by electron withdrawing groups are carried out in solvents of low permittivity weak non-covalent interactions, play a significant role[21-23]. H-bonding and other non-covalent interactions have been also found on ANS under ultrasound irradiation applied to the synthesis of substituted phenylenediamines[24], on the preparation of a corticotropin-releasing factor antagonist[10], and in the synthesis of a new fluorescence probe[11], to mention just a few applications in modern organic synthesis.Nudelman and Palleros 25, 26 proposed three decades ago, that the observed kinetic behaviour of third order dependence on amine (B), is consistent with a new mechanism involving attack of the dimer of the nucleophile (B:B) Eqn. 1, where S and P stand for substrate and products, respectively, and B is shown as DMPA (one of the studied diamines in the present paper).
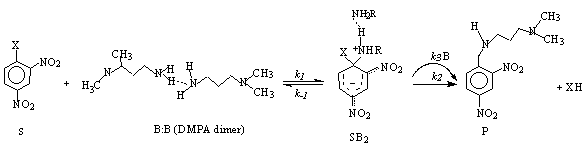 | (1) |
2. Material and Methods
2.1. General Procedures
- UV-VIS spectra were recorded in a Shimadzu UV-VIS 240 graphic printer PR-1 spectrophotometer. 1H and 13C NMR spectra were recorded in a Bruker ARX-300 spectrometer instrument. J values are given in Hz. NMR spectra were determined in CDCl3 as solvent. The IR spectra were recorded in a KBr disc using a Nicolet Nexus FT-IR spectrometer. Thin-layer chromatography was performed on Merck Kiesegel 60 F254. Melting points were determined in a Kofler hot stage and are uncorrected.
2.2. Reagents and Solvents
- Reagents and solvents were purified using previously reported procedures 1.1-(2-aminoethyl)piperidine, (2-AEPip, Aldrich): the commercial product was kept over sodium strings during several days, distilled by reduced pressure fractional distillation over zinc powder and then twice over sodium strings under reduced pressure. The fraction 78-80ºC at 20 mmHg was collected. It was kept in desiccator under dry nitrogen atmosphere, protected from light, and it was re-distilled before usingN-(3-aminopropyl)morpholine, (3-APMo, Aldrich): the commercial product was kept over sodium strings during several days, distilled by reduced pressure fractional distillation over zinc powder and then twice over sodium strings under reduced pressure. The fraction 96-97C at 20 mmHg was collected. It was kept in desiccator under dry nitrogen atmosphere, protected from light, and was re-distilled before usingEthylendiamine (ETDA, Fluka) was kept overnight over potassium hydroxide, distilled over zinc powder and then over sodium; both distillations were carried out at normal pressure, and retrieve the fraction b.p. 116-118ºC (lit. 116.5ºC) 22. It was kept in a desiccator protected from light. 4(5)-2’-Aminoethylimidazole (histamine base, Fluka) was used without any purification and was kept in a desiccator protected from light3-dimethylamino-1-propylamine (DMPA) was prepared from dimethylamine and acrylonitrile, 28. After two days, the excess of dimethylamine was distilled under reduce pressure 75-77ºC/11 mmHg). TheN,N-dimethylpropanenitrile obtained was reduce with Na/EtOH. Distillation of the resulting product gave 3-dimethylamino-1-propylamine as a liquid, which was stored under nitrogen atmosphere at 5ºC. 1H NMR (CDCl3): δ = 1.30 (s, 2H, -NH2); 1.71 (m, 2H, -CH2-); 2.32 (s, 6H, CH3); 2.41 (t, 2H, -CH2-), 2.84 (t, 2H, -CH2-).Aminoethylimidazole (histamine base, Fluka) was used without further purification and was kept in a desiccator protected from light.2-guanidinobenzimidazole, (2-GB, Aldrich) was crystallised twice from ethyl acetate. To assure fully removal of the solvent, the crystals were dissolved in chloroform and vacuum was applied until a dried residue was obtained; it was reduced to powder in a mortar and the procedure was repeated until no impurities were detected by thin-layer chromatography. Finally, it was kept in a desiccator protected from light under dry nitrogen atmosphere (mp 242–244 ºC, lit.[29] 242.8–244.5ºC). IR ν cm_1: 3448, 3210 (N—H), 1648 (C—N), 1600, 1542 (C—C), 1392, 1274(C—N).2,4-dinitrochlorobenzene (DNClB, Sigma), wascrystallized twice from absolute ethanol (mp 52-53°C, lit. 1 52-53°C).2,4-dinitrofluorobenzene, (DNFB, Merck), was distilled at reduced pressure under nitrogen (b.p. 122–123 8C at 5mm Hg, lit.[22] 119ºC at 2 mmHg) and was kept in a desiccator protected from light under dry nitrogen atmosphere.The substitution products were prepared from 2,4-dinitrochlorobenzene and the corresponding amine. In all cases, the compounds were obtained in almost quantitative yields, as yellow crystals. The substitution compounds were characterized as follows:N-(2,4-dinitrophenyl)-1-(2-aminoethyl)piperidine (mp 122-123 ºC), 1H NMR (CDCl3): δ 9.04 (s, 1H), 8.15 (d, 1H), 6.81 (d, 1H), 3.36 (t, 2H), 2.63 (t, 2H), 2.40 (t, 4H), 1.49 (m, 6H), 1.02 (s, 1H). 13C RMN (CDCl3) δ 150.91, 148.50, 147.15, 131.52, 120.77, 115.34, 49.70, 47.90, 43.80, 27.80, 25.90. IR (KBr) ν cm-1: 3480 (N-H), 1530 (N-H), 1540 and 1380, (NO2).N-(2,4-dinitrophenyl)-N-(3-aminopropyl)morpholine (mp 145-146ºC) 1H NMR (CDCl3): δ 9.02 (s, 1H), 8.30 (d, 1H), 7.20 (d, 1H), 3.61 (t, 4H), 3.07 (t, 2H), 2.85 ( t, 2H), 2.21 ( t, 4H), 2.00 (s, 1H), 1.80 (m, 2H).13C NMR (CDCl3): δ 150.91, 148.50, 147.15, 131.52, 120.77, 115.34, 68.10, 51.40, 46.70, 39.90. IR (KBr) ν cm-1: 3520 (N-H), 1635 (N-H), 1510 and 1340 (NO2), 1110 (C-O-C). N-(2,4-dinitrophenyl)ethylenediamine (mp 108-110°C), 1H NMR (CDCl3) δ 2.30 (s, 1H), 8.93 (d, 1H), 6.87 (d, 1H), 8.12 (m, 1H), 3.28 (t, 2H), 3.00 (t, 2H), 1,40 (s, 2H), 13C NMR (CDCl3): δ 40.90, 48.31, 121.51, 121.87, 132.55, 148.10, 148.50, 149.90. IR (KBr) ν cm-1: 3320 (-N-H); 2850 (C-H, -CH2); 1640 (–NH2); 1590 (C-C); 1510 (–NO2); 1470 (C-H –CH2); 1340 (–NO2); 1120 (-C-N); 890 (C-H); 780 (two bands, –NH2); 710 (C-H) .N-(2,4-dinitrophenyl)histamine (mp 158-160C), 1H NMR (CDCl3): δ 12.60 (s, 1H), 9.03 (s, 1H), 8.25 (d, 1H, 3JHH = 9.5 Hz), 8.08 (s, 1H), 7.59 (s, 1H), 7.10 (d, 1H, 3JHH = 9.5 Hz), 3.20 (t, 2H), 3.05 (t, 2H), 2.30 (s, 1H), 13C NMR (CDCl3)ppm: δ 150.91, 148.50, 147.15 ,136.20, 131.52, 122.40, 121.30, 120.77, 115.34, 38.79, 23.19. IR (KBr) ν cm-1: 3450 (N-H); 2750 (C-H, -CH2); 1640 (N-H); 1590 (C-C); 1510 (C-C); 1500 (–NO2); 1455 (C-H, -CH2); 1340 (–NO2); 880 (C-H); 770 (δC-H).3-dimethylamino-1-N-(2,4-dinitrophenyl)propylamine (mp 100-102ºC), 1H NMR (CDCl3): δ 2.10 (s, 1H), 9.30 (d, 1H), 7.40 (d, 1H), 8.48 (m, 1H), 3.70 (t, 2H), 2.01(t, 2H), 2.65 (t, 2H), 2.31 (s, 6H), 13C NMR (CDCl3): δ 27.65, 44.48, 45.40, 58.01, 119.34, 122.35, 128.87, 147.80, 148.48, 149.90. IR (KBr) cm-1: 3320 (N-H); 2850 (C-H, -CH2); 1658 (-N-H); 1500 ( –NO2); 1470 (-C-H, -CH2); 1340 (–NO2); 1380 ( C-H, –CH3); 1120 (C-N); 860 (-C-H); 740 (C-H).[1-N-(2-benzimidazol)-3-N-(2,4-dinitrophenyl)guanidine (mp 218–220 ºC), 1H NMR (DMSO-d6): d 11.12 (s, 1H), 9.03 (s, 1H), 8.25 (d,1H), 7.20 (m, 2H), 7.10 (d, 1H), 6.92 (m, 2H), 2.30 (s, 1H), 2.10 (s, 2H). 13C NMR (DMSO-d6): d 160.00, 152.00, 149.90, 148.48, 147.80, 140.80, 128.87, 125.80, 123.70, 121.30, 120.30, 119.34, 114.34, 110.20. IR (KBr) ν cm-1: 3439 and 3196 (N—H),. 1640 (N—H) and (NH2), 1626 (C—N), 1510, (NO2), 1340, (NO2), 780 (NH2)].
2.3. Kinetic Procedures and Ancillar Spectrophotometric Measurements
- UV-VIS spectra of the substrates, the products, and different mixtures of both compounds with the amine in toluene at several concentrations were recorded in a Shimadzu UV-VIS 240 graphic printer PR-1 spectrophotometer. The extinction coefficients of the products were determined at λ max and at λ = 450 and 400 nm; at that wavelengths the reagents are transparent under these conditions. All the solutions were found to obey Beer’s law. Kinetic runs were performed by the methods previously reported 22, following the appearance of the reaction product at λ = 450 or 400 nm. The reactions of DNClB and DNFB with ethylendiamine, 3-dimethylamino-1-propylamine, 1-(2-aminoethyl)piperidine and N-(3-aminopropyl) morpholine were recorded directly in the thermostated cell of the spectrophotometer at 25 ± 0.2°C. The reactions of the same substrate with histamine and2-guanidinobenzimidazole were carried out in sealed ampoules (under nitrogen) at 40 ± 0.2°C. In all cases pseudo-first-order rate kinetics were observed. The absorption spectrum of the reaction mixture at “infinite time” corresponded within ±2 % with the “theoretical” value calculated from application of Beer’s law to solutions of the product independently prepared in the desired solvent. Pseudo-first-order coefficients, kΨ, were obtained by the least-squared method as the slope of the correlation ln (A∞ - At )/ A∞ against time, where A∞ is the optical density of the reaction mixture measured at “infinity” (more than ten half-lives).
3. Results and Discussion
- One of the easily determined feature of the “dimer nucleophile” mechanism is the forth order kinetics (third order in amine); this has been mostly observed in substrate nucleophile systems, where departure of the nucleofuge is the rate determining step. In Nudelman’s mechanism, the intermediate SB2 is highly zwiterionic and the extra amine molecule stabilizes the developing charges in non-polar aprotic solvents. The proposed mechanism does not preclude attack by the monomer, as shown in the three step pathway depicted below in Scheme 1. Nevertheless, due to the electron density on the hydrogen-bonded nitrogen, the H-bonded amines (forming inter- or intramolecular homo-aggregates) are better nucleophiles than monomeric amines12. This arouse the concern why “dimer nucleophiles” are not observed when the first step is rate determining?.
 | Scheme 1. Overall reaction diagram including equilibrium between the intermediates SB, SB2 and HBA:B |
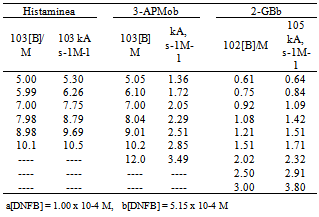
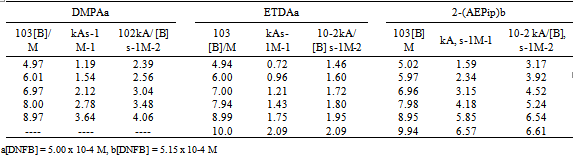
3.1. Reactions of Di- and Polyamines with DNFB in Toluene (Second Step Rate Determining)
- The kinetics of the reactions of DNFB with: 1-(2-aminoethyl)piperidine, 2-(AEPip)[1],N-(3-aminopropyl)morpholine,[1] 3-(APMo), ethylendiamine(ETDA)[15],3-dimethylamino-1-propylamine (DMPA)[22],histamine[22] and 2-guanidinobenzimidazole (2-GB), were studied in the presence of variable amounts of the nucleophile. Tables 1 and 2 shows the observed results for the reactions with DNFB: the bimolecular rate coefficient kA and the ratio kA/[B] are given. For 2-(AEPip), ETDA and DMPA the second-order rate coefficients, kA, were found to increase rapidly with amine concentration,[B]; the plot of kA vs[B], (not shown), illustrates a quadratic dependence, while the quotient kA/[B] plotted against[B] is a straight line. This result is consistent with a third-order-in-amine term in the kinetic law, which has been observed previously in other systems,[12, 16, 25-26] and can be interpreted by the mechanism shown in Eqn. (2), where the dimer (B:B) of the nuc1eophile attacks the substrate S to form the intermediate SB2; then a third molecule of amine assists the intermediate in the decomposition step. The simplified form of kinetic law is given by Eqn (3), where K =[B:B]/[B] stands the amine self-association constant.
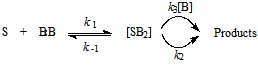 | (2) |
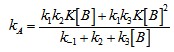 | (3) |
|
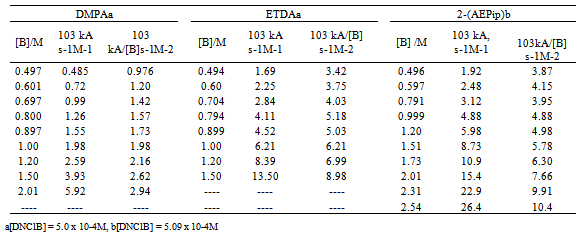
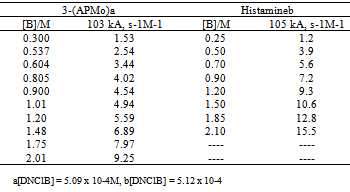
3.2. Reactions of Diamines with DNClB in Toluene (First Step Rate Determining)
- The kinetics of the reactions of DNClB with the 2-(AEPip), 3-(APMo), ETDA, DMPA, and histamine were studied in the presence of variable amounts of the nucleophile. Tables 3 and 4 shows the observed results for the reactions with DNClB: the bimolecular rate coefficient kA and the ratio kA/[B] are given.
|
|
3.3. Derivation of the Whole Kinetic Law for Third Order in Amine when First Step is Rate Determining
- The whole kinetic expression for the second order coefficient, kA, when the second step is rate-determining, as well as the simplification that can be applied to limiting situations were previously discussed. The expression for kA considering only the attack by the dimer can be reduced to equations 2, 3 where K is the equilibrium constant for the monomer:dimer equilibrium.ANS reactions carried out in aprotic solvents using mono-[16, 21] and polyfunctionalized amines[1, 22] able to form intra- or intermolecular hydrogen bond with good leaving groups substrates also leads to atypical finding, and the “dimer nucleophile” mechanism is very well established in these systems. Initially, most of the systems in which third-order in amine kinetic law was observed were performed using poor nucleofuge substrates[25, 26]; in the last years we reported evidences for this atypical kinetic behavior observed also with a good nucleofuge substrate[1, 21-23].It is very well accepted that in ANS reactions of amines with good nucleofuges substrates, such as DNClB, the first step is the rate limiting. To analyze this situation, involving attack by the dimer and the monomer, it should be considered that: (a) the first step is slower than the second one and (b) that the dimer is better nucleophile than the monomer. Application of the steady-state treatment to the whole mechanism for the dimer and monomer leads to Eqn. 4, that involves the specific rate constants for each step, the association equilibrium for the nucleophile, K1, and the constant for the equilibrium between the intermediates SB and SB2, K2 (Scheme 1).
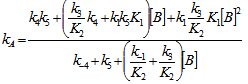 | (4) |
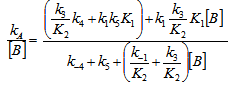 | (5) |
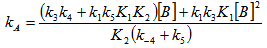 | (6) |
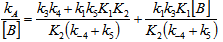 | (7) |
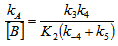 | (8) |
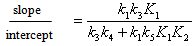 | (9) |
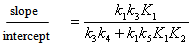 | (10) |
3.4. Catalysis by Hydrogen-Bond Acceptor (HBA) Additives in ANS Reactions Carry Out in Aprotic Solvents
- Overwhelming evidence of the participation of mixed aggregates between the amine nucleophile and HBA additives[12, 21, 31], HBA co-solvents[1, 12, 16, 19, 20, 32] on the rates of ANS has been accumulated in the literature. When the reactions are run in presence of HBA additive, such as tertiary non nucleophilic amines[21, 31] or in the presence of small amounts of HBA solvent[1, 12, 16], a new mixed associated nucleophile, B:HBA, is present in the system as depicted in Scheme 1. Three competing nucleophile reactions can occur: attack by the dimer (measured by k1), by the monomer (determined by k4), and by the B:HBA aggregate (measured by k7). An equilibrium between the three possible tetrahedral intermediated SB, SB2 and SB:HBA is established (measured by the equilibrium constants K2,, K3 and K4, respectively) as depicted in Scheme 1.Defining the partial rate constants as:
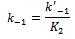
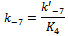
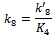 where:
where: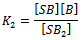 and
and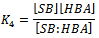 By application of the steady-state hypothesis for intermediates SB, SB2 and SB2:HBA, the following kinetic expression is obtained, Eqn 11:
By application of the steady-state hypothesis for intermediates SB, SB2 and SB2:HBA, the following kinetic expression is obtained, Eqn 11: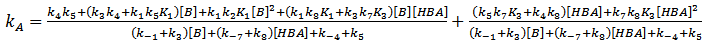 | (11) |
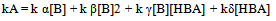 | (12) |
4. Conclusions
- The ANS reactions with amines in aprotic solvents pose various difficulties, related to the inability of those solvents to stabilize ionic species. Overwhelming evidence on the rol of homo- and heteroaggregates of the nucleophile in solvents of low permittivity, has been afforded, and the “dimer mechanism” is currently well settled for ANS carried out with poor nucleofugues substrates.The present paper affords kinetic evidence and derivation of the whole kinetic expressions for ANS that allow to conclude these reactions can be considered well settled in aprotic solvent. Rationalizations of the involved mechanisms are based on the strong H-bond interactions between the it-self nucleophile and nucleophile:HBA additive or nucleophile: HBA co-solvent operating as an entity, consistent with the experimental evidence.Due to the higher electron density on the hydrogen-bond donor nitrogen, hydrogen bonded amines are better nucleophiles than those in which no hydrogen-bonding interactions are possible. So, the reactions with intermolecular homo- or HBA: nucleophile aggregates are faster than with the non hydrogen-bonded nucleophile. The intramolecular hydrogen bond formation in the nucleophile significantly reduces or prevents the formation of intermolecular dimers, thus, the hydrogen bonded nucleophile structure is crucial. Polyamines where internal hydrogen bond is expected are prone to react in the monomeric state under conditions that favor the dimer mechanism, which is interpreted by the formation of an “intramolecular dimer”; while those in which no intramolecular hydrogen bond is possible, react by an intermolecular homo- or heterodimer.These new findings can contribute to show alternative pathways and/or more efficient routes to industrial chemical processes using ANS, as it is demonstrated by the current extensive literature
ACKNOWLEDGEMENTS
- The authors gratefully acknowledged financial support from the Universidad Nacional del Comahue (grant nº I183-UNCo) and from the Agency for the Promotion of Science and Technological Research (ANPCYT) from Argentine (grant n° PICT 2007/0347).
References
| [1] | Alvaro, C. E. S., Ayala, A. D., and Nudelman, N.S, 2011, Hydrogen-bonded nucleophile effects in ANS. Reactions of 1-chloro and 1-fluoro-2,4-dinitrobenzene with2-guanidinobenzimidazole, 1-(2.aminoethyl) piperidine andN-(3-aminopropyl)morpholine in aprotic solvents, J. Phys. Org. Chem., 24 (2), 101-109. |
| [2] | Harifi-Mood, A. R., Rahmati, M. and Gholami, M. R., 2011, Solvent polarity and hydrogen bond effects on nucleophilic substitution reaction of 2-bromo-5-nitrothiophene with piperidine. Int. J. Chem. Kinet, 43,185–190. |
| [3] | Jamali-Paghaleh, J, Harifi-Mood, A. R. and Gholami, M. R., 2011, Reaction kinetics investigation of 1-fluoro- 2,4-dinitrobenzene with substituted anilines in ethyl acetate–methanol mixtures using linear and nonlinear free energy relationships, J. Phys. Org. Chem., 24 (11), 1095-1100. |
| [4] | Basilio, N., García-Río, L., Peña-Gallego, A. and Pérez-Lorenzo, M., 2012, Molecular recognition-based catalysis in nucleophilic aromatic substitution: a mechanistic study,Affiliation Information New J. Chem., 36, 1519-1526. 1. Center for Research in Biological Chemistry and Molecular Materials (CIQUS),University of Santiago de Compostela, 15782 Santiago de Compostela, Spain |
| [5] | Crampton, M. R. 2011, “Nucleophilic Aromatic Substitution” in Organic Reaction Mechanisms: An annual survey covering the literature dated January to December 2009.A. C Knipe, Ed. Chichester, UK: John Wiley & Sons, Ltd., chapter 5, pp 175-187, and references cited therein |
| [6] | Fathalla, M. F., 2012, Kinetics of the reaction of 2-Chloro-3,5-dinitro-benzotrifluoride with anline in toluene and methanol-toluene mixed solvents, Chin. J. Chem., 30, 109-114. |
| [7] | Capitillo, D., Chinea, C., and Cabrera, G., 2008, Síntesis y funcionalización de un copolímero de estireno y divinilbenceno y su posible aplicación como soporte en la síntesis en fase sólida, Revista Iberoamericana de Polímeros, 9 (3), 294-299. |
| [8] | Seifert, A., Ladewig, K., Schönherr, P., K. Hofmann, K., Lungwitz, R. Roth, I., Pohlers, A., Hoyer, W., Baumann, G., Schulze, S., Hietschold, M., Moszner, N., Burtscher, P., and. Spange, S., 2010, Synthesis of dye functionalized xerogels via nucleophilic aromatic substitution of fluoro aromatic compounds with aminosilanes, J. Sol-Gel Sci. Technol. 53 (2), 328-341. |
| [9] | Angell, Y., Chen, D., Brahimi, F., Uri Saragovi, H. and. Burgess, K., 2008, A combinatorial method for solution-phase synthesis of labeled bivalent â-turn mimics, J. Am. Chem. Soc., 130, 556-565. |
| [10] | Caron, S. , Do, N. M., Sieser, J. E., Whritenour, D. C. and Hill, P. D., 2009, Preparation of a corticotropin-releasing factor antagonist by nucleophilic aromatic substitution and copper-mediated ether formation, Org. Process Res. Dev., 13 (2), 324–330. |
| [11] | Shibata, H. Abe, M. Ito, Y. Kondo, S. Shimizu, K. Aikawa and Y. Ito, 2009, DNA templated nucleophilic aromatic substitution reactions for fluorogenic sensing of oligonucleotides, Chem. Commun., 6586-6588. |
| [12] | N. S. Nudelman, “SNAr Reactions of Amines in Aprotic Solvents” in The chemistry of amino, nitroso, nitro and related groups, Supplement F2, S. Patai, Ed. London: Wiley J & Sons Ltd, 1996, chapter 29, pp 1215–1300, 1996. |
| [13] | F. Terrier, “Nucleophilic aromatic displacement: the influence of the nitro group”, in Organic nitro chemistry series, H. Ferrer, Ed. New York: VCH Publishers: Weinheim, 1991. |
| [14] | R. W. Gillham, “Cleaning halogenated contaminants for groundwater” U.S. Patent 5266213. Nov. 30, 1993, and references cited therein. |
| [15] | C. Reichardt, “Solvent and Solvents Effects” in Organic Chemistry, 4td Ed., Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, 2011. |
| [16] | Alvaro, C. E. S., Nudelman, N.S, 2003, Unusual solvent effects in the reactions of 1-halo-2,4-dinitrobenzenes and aniline in aprotic and dipolar-aprotic solvents. Effects of aggregates. ARKIVOC, Part. (x), 95-106. |
| [17] | Fathalla, M. F., 2011, Kinetics of the reaction of 2-chloro-quinoxaline with hydroxide ion in ACN–H2O and DMSO–H2O binary solvent mixtures, J. Solution Chem., 40, 1258–1270. |
| [18] | Asghar, B. H. M., Fathalla, M. F., Hamed, E. A.,2009, Solvent and Substituent Effects on the Reaction of 2-and 4-Chloro-3,5-dinitrobenzotrifluorides with Substituted Anilines, Int. J. Chem. Kinet., 41 (12), 777 - 786. |
| [19] | Mancini, P. M. E., Fortunato, G., Adam, C., Vottero, L. R., and Terenzani, A., 2002, Specific and non-specific solvent effects on aromatic nucleophilic substitution. Kinetics of the reaction of 1-fluoro-2,6-dinitrobenzene and homopiperidine in binary solvent mixtures. J. Phys. Org. Chem., 15 (5) 258–269, and references cited therein. |
| [20] | Sung, R. Y., Choi, H. ., Lee, J. P., Park, J. K., Yang, K. and I. S. Koo, 2009, Kinetic studies on the nucleophilic substitution reactions of 4-X- substituted-2,6-dinitrochlorobenzene with pyridine in MeOH-MeCN mixtures. Bull. Corean Chem. Soc., 30 (7) 1579-1582. |
| [21] | Nudelman, N. S., Alvaro, C. E. S. and Yankelevich, J. S., 1997, Aggregation effects in the reactions of 2,4-dinitrochlobenzene with aniline in aprotic solvents, J. Chem. Soc., Perkin Trans. 2, 2125-2130. |
| [22] | Alvaro, C. E. S., Nudelman, N. S., 2005, Role ofhydrogen-bonded nucleophiles in aromatic nucleophilic substitutions in aprotic solventes. Reactions of halonitrobenzenes with ethylenediamine,3-dimethylamino-1-propylamine and histamine in toluene, J. Phys. Org. Chem., 18 (8) 880-885. |
| [23] | Alvaro, C. E. S., Nudelman, N. S., 2011, The crucial role of H-bonding in the mechanisms of reactions with diamines in aprotic solvents, Trends in Org. Chem. 15, 95-107. |
| [24] | Raouafi, N., Belhadj, N., Boujlel, K., Ourari, A., Amatore, C., Maisonhaute, E., and Bernd, B., Schöllhorn, 2009, Ultrasound-promoted aromatic nucleophilic substitution of dichlorobenzene iron(II) complexes, Tetrahedron Lett., 50 (15), 1720-1722. |
| [25] | Nudelman, N. S. and Palleros, D., 1983, Reactions of Nitroanisoles. Part. IV. The Reactions of 2,4 and 2,6Dinitroanisoles with Cyclohexylamine. Evidence for a "Dimer" Nucleophile", J. Org. Chem., 48, (10), 1607-1612. |
| [26] | Nudelman, N. S. and Palleros, D., 1983 The Reaction of 2,4Dinitrofluorbenzene with oAnisidine in Benzene, Further Evidence of the "Dimer" Mechanism". J. Org. Chem., 48 (10), 1613-1617. |
| [27] | M. B. Smith, and J. March, March’s Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 6th ed., Wiley-Interscience, John Wiley & Sons, Inc.,Hoboken, New Jersey, pp 856-857, 2007. |
| [28] | A. Vogel, Practical Organic Chemistry, 5th ed.; Longman, Inc: New York, USA, 1991. |
| [29] | Andrade-López, N., Ariza-Castolo, A., R. Contreras, Vasquez-Olmos, A., Barba Behrens, H., and Tlahuext, H.,1997, Versatile behaviour of 2-guanidinobenzimidazole nitrogen atoms towards protonation, coordination and methylation, Heteroatom Chem., 8, 397–410. |
| [30] | Nudelman, N. S., Alvaro, C. E. S., 2011, Inter- and intramolecular hydrogen bonds in polyamines:variable-concentration 1H-NMR studies, J. Phys. Org. Chem., 24, (11), 1067-1071. |
| [31] | Nudelman, N. S., Alvaro, C. E. S., Nicotra, V. and Yankelevich, J. S., 1999, Effects of the nucleophile structure on the mechanisms of reaction of 2,4-dinitrochlorobenzene with aromatic amines in aprotic solvents. Coll. Czech. Chem. Com. 64, 1583-1593. |
| [32] | Durantini, E., Zingaretti, L., Anunziata, J. D. and Silber, J. J., 1992, Solvent effects in aromatic nucleophilic substitution reactions in non-polar aprotic solvents. Inhibition by electron-donor–acceptor (EDA) complexation of the substrate by aromatic solvents. J. Phys. Org. Chem., 5 (9) 557–566 and references therein. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML