-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2013; 3(1): 21-28
doi:10.5923/j.pc.20130301.04
Suzuki Chemistry-A Promising Ligand-Free Metal Catalyst System in Situ Generated from PdII Supported on MgO
Lin Huang , Fengxi Chen , Yu Wang, Pui Kwan Wong
Heterogeneous Catalysis, Institute of Chemical and Engineering Sciences, A*STAR, Singapore, 627833, Singapore
Correspondence to: Lin Huang , Heterogeneous Catalysis, Institute of Chemical and Engineering Sciences, A*STAR, Singapore, 627833, Singapore.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ligand-free Pd catalyst systems derived from Pd(acac)2/Mg(OH)2 and their catalytic properties toward the Suzuki coupling of bromobenzene and phenylboronic acid have been studied. Catalytic study illustrates that the catalyst system (0.2 mol% Pd) in situ generated in the Suzuki reaction is highly active in a mixture of DMA and H2O and in H2O alone at 22-50 ℃, and can be recycled at least three times. The catalysis heterogeneity tests by filtration test and catalyst poisoning with solid-bound thiols reveal that soluble leached Pd from Pd/MgO is fully responsible for the catalytic activity. Transmission electron microscopy measurements show that Pd0/MgO has a smaller mean Pd particle size than Pd0/SiO2. The relation between the size of the supported Pd particles and the catalytic activity further suggests that the size of supported Pd particles determines catalytic activity in a C-C coupling reaction although the catalysis is homogeneous in nature.
Keywords: Suzuki Reaction, In Situ Generated, Palladium, Magnesia, Supported
Cite this paper: Lin Huang , Fengxi Chen , Yu Wang, Pui Kwan Wong , Suzuki Chemistry-A Promising Ligand-Free Metal Catalyst System in Situ Generated from PdII Supported on MgO, Physical Chemistry, Vol. 3 No. 1, 2013, pp. 21-28. doi: 10.5923/j.pc.20130301.04.
Article Outline
1. Introduction
- Catalytic C-C couplings such as Heck-, Suzuki-, Stille- and Sonogashira-type reactions are a well-established methodology in modern organic synthesis. The coupling products are of considerable interest for the production of fine chemicals. Among these C-C coupling reactions, Suzuki reactions are the most widely used in industry.1-3 Suzuki reactions not only employ accessible substrates and proceed under mild conditions, but also offer the additional advantages of being largely unaffected by the presence of water, tolerating a broad range of functionality, and yielding nontoxic byproducts. Suzuki couplings of aryl and vinyl halides/triflates with boronic acids are applied to the production of compounds such as losartan, a Merck antihypertensive drug. This popularity is attributable to a variety of factors, such as the commercial availability of a large number of boronic acids, as well as their nontoxic nature and stability to heat, air and moisture.Homogeneous transition-metal catalyzed C-C coupling reactions are extensively studied because of its tolerance of reactive functional groups and high degree of selectivity. The activity and selectivity of these homogeneous metal catalysts can be readily optimized by modifying the metal center with a variety of organic ligands. A major difficulty of using these coupling reactions in the fine chemical industry is contamination of the products by traces of toxic catalyst residue or colloidal metallic particle formed during the reactions.Ligand-free catalyst systems are viewed to be promising for C-C coupling reactions from both operational and economic points of view.4-8 Since ligand-free Suzuki couplings were first reported by Wallow and Novak in 1994,5 a great number of advances have been made in the development of ligand-free Pd catalyzed Suzuki couplings.2 Quite a number of studies on inorganic material-supported Pd particle catalysts such as Pd/SiO2, Pd/SBA-15, Pd/MCF, Pd/PMO, Pd/zeolite Y, Pd/Al2O3, Pd/MgO, Pd/CeO2, Pd/ZrO2, Pd/Fe2O3, Pd/BaSO4, Pd/C, Pd/LDH and Pd/polymer for Suzuki couplings have been documented.2 In the present article, we intend to report a study regarding an effective ligand-free Suzuki coupling catalyst system in situ generated from PdII/MgO. The reaction involves the coupling of bromobenzene (PhBr) and phenylboronic acid (PhB(OH)2) under an aqueous condition in the presence of Na2CO3 at 50℃, as shown in Scheme 1. We illustrate the obvious advantages of Pd/MgO over Pd/SiO2, PdII/MgO over conventional Pd0/MgO in catalytic performance. We correlate the high activity of such a catalyst system to the size effect of supported metal particles. Besides, in order to reveal the homogeneity or heterogeneity of catalysis, we present compelling evidence for the nature of the true catalytic species for the Suzuki reaction in virtue of control experiments such as filtration test and catalyst poisoning by solid-bound thiols. To our knowledge, the present contribution reports the second case of MgO as a catalyst support for use in Suzuki reactions after the study of Kantam et al. published in 2005.9
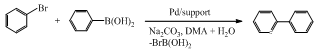 | Scheme 1. Suzuki coupling of PhBr and PhB(OH)2 with Pd/support |
2. Results and Discussion
2.1. Behavior of Supported Pd Precatalysts in Suzuki Reactions
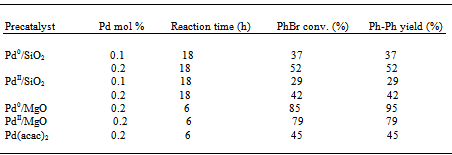
- As silica gel (SiO2) is a neutral, typical support for heterogeneous catalysts, we began our study of Suzuki reactions with SiO2-supported Pd precatalysts. Figure 1 shows the variations of the activities of conventional Pd0/SiO2 with N,N-dimethylacetamide (DMA): H2O volumetric ratio in the coupling of PhBr and PhB(OH)2 in the presence of Na2CO3 at 22oC. From the PhBr conversion or biphenyl (Ph-Ph) yield profiles, the pure DMA or H2O as a solvent was unfavorable for the Suzuki reaction, the mixed DMA and H2O at a molar ratio of 1 : 1 brought about an optimal PhBr conversion or Ph-Ph yield. The results seem to account for the dependence of efficiency of the Suzuki reaction on the solubility of substrates and catalyst in a solvent used. The DMA : H2O = 1 : 1 molar ratio appears to be the best condition for the Suzuki reaction.
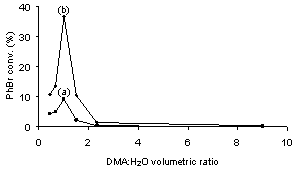 | Figure 1. PhBr conversions as a function of DMA : H2O volumetric ratio in the coupling of PhBr and PhB(OH)2 over Pd0/SiO2 (0.1 mol% Pd) at 22 ℃ for (a) 1 h; (b) 18 h |
|
|
|
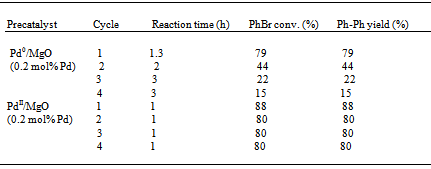
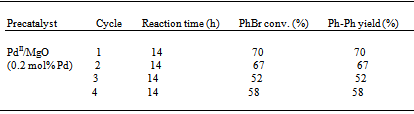
- Afterwards, we extended our work to exploring the Suzuki reaction with PdII/MgO in H2O alone. Reactions conducted in H2O alone are attractive as H2O is nontoxic, nonflammable, inexpensive and is easily separated from organic products. In Table 3 are presented the preliminary results of the Suzuki reaction with PdII/MgO in H2O alone at 50 ℃. Compared to the reaction in the mixture of DMA and H2O, the reaction in H2O alone required a very long time to reach a PhBr conversion or Ph-Ph yield of over 50 %. Nonetheless, PdII/MgO is still a promising catalyst system in H2O alone, because it can be recycled at least three times with satisfactory Ph-Ph yields. The lower catalytic activity can be caused by two reasons. One is the limited solubility of substrates in H2O alone, the other the limited amount of Pd0 formed due to the more difficult reduction of PdII by the reaction media using H2O alone as the solvent.
2.2. Nature of the True Catalytic Species for Suzuki Reactions with Supported Pd Particles
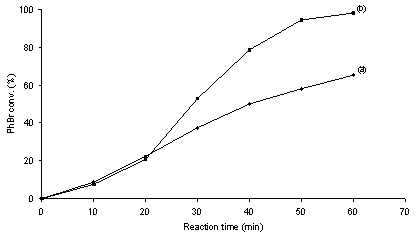
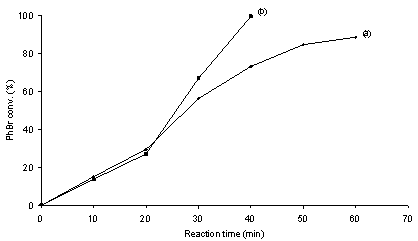
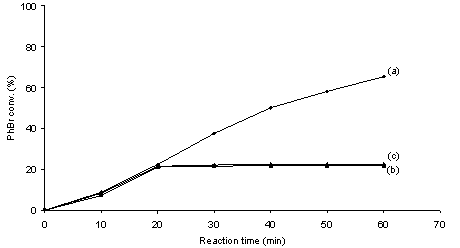
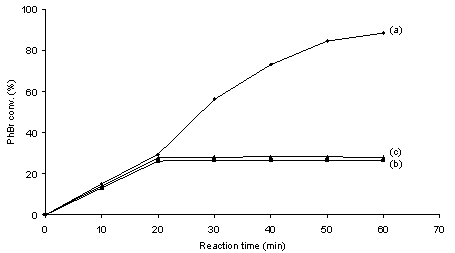
- It is generally admitted that metal leaching into solution from supported metal particle precatalysts occurs inevitably as long as C-C coupling reactions proceed on single metal centers following the M0/MII catalytic cycle.10 The occurrence of leaching of metal species into solution during C-C coupling reactions with heterogeneous precatalysts leads to an ambiguous understanding on the nature of catalysis as well as catalyst contamination towards the products.1,10 It is known that ppm or even ppb levels of soluble Pd are sufficient to produce high yields of C-C coupling products.3,11,12 Therefore, identifying the true catalyst is critically important for advances in the rational design of C-C coupling catalysts. The filtration test and catalyst poisoning are most powerful control experiments for probing the occurrence of homogeneous catalysis.1,10Figures 2 and 3 show the coupling profiles of PhBr and PhB(OH)2 in a mixture of DMA and H2O (1 : 1 molar ratio) over Pd/MgO and over filtrates at 50 ℃. In the cases of both Pd0/MgO and PdII/MgO, the filtrates after 20 min of reaction continued to catalyze the reaction and led to markedly higher conversions. The positive of the filtration tests suggests that soluble leached Pd species are catalytically active for the Suzuki reaction. The observed difference in activity between over the filtrates and over Pd/MgO can be interpreted in terms of the effect of supported metal particles on the catalysis of soluble metal.13 To further test catalysis homogeneity, the catalyst poisoning is often applied as a critical mean to prove whether soluble species take part in catalysis in conjunction with the filtration test.1,10 Figures 4 and 5 show the poisoning effect of MPA and SH-SiO2 on the catalytic activity of the Pd/MgO system in the Suzuki reaction at 50 ℃. In the cases of both Pd0/MgO and PdII/MgO, after addition of either of the poisons, the catalytic activity the Pd/MgO system was rapidly quenched. There was no longer growth of PhBr conversion with reaction time in the presence of either of the poisons. This unambiguously indicates that all of the possible catalytic species responsible for the Suzuki reaction are effectively poisoned by MPA or SH-SiO2. Not only consistent with the filtration test, the results also demonstrate that soluble leached Pd is solely responsible for the Suzuki reaction because MPA or SH-SiO2 is a selective poison of soluble Pd species.14The nature of the catalytic species for ligand-free Suzuki reactions with supported Pd particles has been extensively studied by many researchers.15-22 Although in some cases supported Pd particles have been claimed to be simply reservoirs of soluble active species,15,17,18,22 in some other cases supported Pd particles have been asserted to contribute to catalysis.16,19-22 Among these studies reported, Schmidt et al. used a simple method based on analysis of the phase trajectories of competing reactions of different substrates to distinguish between homogeneous and heterogeneous catalysis in Suzuki and Heck reactions.22 Although the dependence of the rate ratio of competing reactions on the quantity of active species is questionable, their kinetic results hypothesized that Suzuki reactions of aryl bromides proceed via both homogeneous and heterogeneous catalytic mechanisms, while Suzuki reactions of aryl iodides take place via homogeneous catalytic mechanism. Strongly evidenced from the filtration test and catalyst poisoning, our study shows the homogeneous nature of the active species in the coupling of PhBr and PhB(OH)2 over Pd/MgO in agreement with the cases reported for Suzuki reactions over Pd/C, Pd/graphite oxide and Pd/Au-S.15,17,18
2.3. Supported Pd Particle Sizes with Regards to Suzuki Reactions
- Our recent and above studies have given some insights into the nature of the true catalytic species for Heck and Suzuki reactions over supported Pd particles.13,14 We have suggested that such catalysis occurs via an Ostwald ripening mechanism.23 As a matter of fact, soluble leached Pd from supported Pd particles is fully responsible for the catalysis.13,14 Supported Pd particles are merely a precatalyst or reservoir to liberate active species into solution. With supported Pd particles, a C-C coupling reaction likely occurs in solution following a “release and catch” mechanism.1 Catalytic Pd species are leached out of supported Pd particles, then catalyze the coupling reaction in solution, and redeposit on Pd0/support at the end of the catalytic cycle. The smaller are supported Pd particles, the more easily they leach into solution and the higher is resulting catalytic activity. Therefore, the size of supported Pd particles determines the catalytic activity of such a catalyst system although the catalysis is homogeneous in nature. Now let us analyze a few different supported Pd catalyst systems with different Pd particle sizes which correspond to their distinct catalytic activities in the Suzuki reaction.Figure 6 illustrates the comparative TEM micrographs and Pd particle size distributions of conventional Pd0/SiO2 and conventional Pd0/MgO. For conventional Pd0/SiO2, the Pd particle size distribution was centered around 9 nm, covering over a wide range of 2-20 nm. By contrast, for conventional Pd0/MgO, the Pd particle size distribution showed a coverage in the range of 2-20 nm with a maximum at 6 nm. The comparative observations show that conventional Pd0/MgO has an obviously smaller mean Pd particle size than conventional Pd0/SiO2. The smaller mean Pd particle size can be correlated with the markedly higher catalytic activity of the Pd0/MgO system for the Suzuki reaction. As for the cases of PdII/SiO2 and PdII/MgO, we have recently reported that Pd0/SiO2 in situ generated from PdII/SiO2 in the Heck coupling of PhBr and styrene at 135 ℃ has much finer Pd particles than conventional Pd0/SiO2.23 It is deemed that Pd0/SiO2 and Pd0/MgO in situ produced from PdII/SiO2 and PdII/MgO in the Suzuki coupling of PhBr and PhB(OH)2 at 50℃ likewise have much smaller Pd particles than conventional Pd0/SiO2 and conventional Pd0/MgO, respectively. This can be supported by the comparative recycling results of Pd0/MgO and PdII/MgO in the Suzuki reaction (Table 2). The much better recycling result of PdII/MgO than that of Pd0/MgO hints that the PdII/MgO system has smaller mean Pd particle size in distribution than the Pd0/MgO system. But at this point, the effects of chemical and structural properties of support on the catalytic activity cannot be ruled out.
3. Conclusions
- The following conclusions can be drawn from the present work:(1) PdII/MgO is a promising catalyst system for the Suzuki coupling of PhBr and PhB(OH)2. The reaction can be carried out at 50 oC in DMA + H2O in high Ph-Ph yields and in H2O alone in satisfactory Ph-Ph yields. The catalyst system (0.2 mol% Pd) can be recycled in DMA + H2O at least three times without loss of activity, and in H2O alone at least three times with satisfactory activity. (2) The catalysis for the Suzuki reaction over PdII/MgO or conventional Pd0/MgO is homogeneous in nature. Soluble leached Pd is solely active for the Suzuki reaction.(3) The catalytic activity of such a supported Pd system is more likely associated with the size of the supported Pd particles. The smaller are supported Pd particles, the higher is resulting catalytic activity.
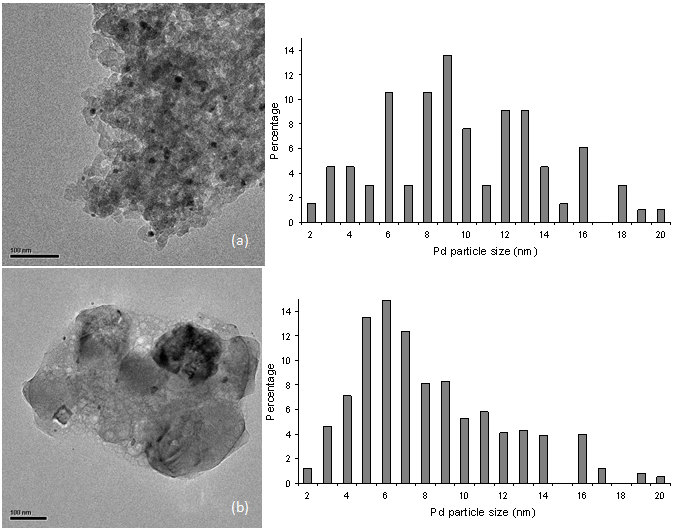 | Figure 6. TEM images and Pd particle size distributions of (a) conventional Pd0/SiO2; (b) conventional Pd0/MgO |
4. Experimental
- SiO2 (Merck grade 10184) having a surface area of 300 m2/g, Mg(OH)2 (95 %) having a surface area of 80 m2/g, MPA (1.5 mmole S/g), SH-SiO2 (1.2 mmole S/g), Pd(acac)2 (99 %), PhBr (99 %), PhB(OH)2 (97 %), anhydrous Na2CO3 ( ≥ 99.5 %), anhydrous DMA (99.8 %) and anhydrous toluene (99.8 %) were purchased from Sigma-Aldrich. The gases H2 and Ar had a purity of 99.999 %.Supported Pd precatalysts were prepared as follows: A support (SiO2 or Mg(OH)2, 1.0 g) was predehydrated at 400oC in flowing purified air for 8 h, and impregnated with a toluene (5 ml) solution of Pd(acac)2 (0.029 g) under Ar. Note that Mg(OH)2 actually decomposed to result in MgO after the thermal treatment at 400oC. The impregnated system was stirred for 2 h under Ar followed by evacuation of the solvent to give dry Pd(acac)2/support containing 1.1 % Pd. PdII/support and conventional Pd0/support were obtained from Pd(acac)2/support by calcination in flowing purified air at 400 ℃ for 8 h and by reduction in flowing H2 at 400 ℃ for 2 h, respectively. A heating rate of 10 ℃/min was used from 22 to 400 ℃ in both cases. Suzuki reactions were conducted at 22-50 ℃ in glass flasks under Ar. In a typical experiment with 0.1-0.2 mol % Pd with respect to PhBr, to a 50 ml three-neck flask equipped with a septum were introduced 10 mmol of PhBr, 12 mmol of PhB(OH)2, 5 mmol of Na2CO3, 0.1- 0.2 g of heterogeneous precatalyst (Pd0/support or PdII/support) or an equivalent amount of homogeneous precatalyst Pd(acac)2 under Ar. Then 10-20 ml of solvent (10 ml of DMA plus 10 ml of H2O, or 10 ml of H2O) was added. After the mixture had been stirred at room temperature for 10 min under Ar, the flask was placed in a preheated oil bath with vigorous stirring (500 rpm). The reaction mixture was sampled at the reaction temperature and atmosphere through a 0.45 µm Whatman syringe filter. In all the reactions run, Ph-Ph was observed as the only product. In a filtration test, the reaction solution was drawn off through 0.45 µm Whatman syringe filters at reaction temperature and atmosphere, and transferred into another flask having Na2CO3 (5 mmol) under Ar. The reaction was quickly initiated with the filtrate under equivalent conditions. In a catalyst poisoning experiment, MPA or SH-SiO2 (0.08 g) was added as the poison to a reaction system at reaction temperature and atmosphere. The atomic ratios of S on the poisons to Pd in the reaction system were 16 : 1 for MPA and 13 : 1 for SH-SiO2, respectively. The S : Pd atomic ratios used in this work were high enough to scavenge removable Pd in solution.14 The Suzuki reactants and products in the reaction samples were analyzed by gas chromatography on a Perkin-Elmer Clarus 500 gas chromatograph with a J&W DB-1 capillary column (30 m x 0.320 mm x 1.00 µm) and a flame ionization detector. The Pd contents in solution and solid samples were determined by the inductively coupled plasma (ICP) technique on a Varian Vista-MPX CCD simultaneous ICP-OES spectrograph. The quantitative analysis of leached Pd from supported Pd into solution was previously described elsewhere. For catalyst recycling, a solid sample containing a used supported catalyst was filtered off from the reaction mixture in air after a reaction cycle had ceased, and washed with DMA. Then it was directly transferred into a clean flask for the following reaction cycle.The microscopic images of supported Pd particles were observed by means of TEM on a JEOL TecnaiG2 microscope. The Pd particle size distributions were determined by counting the sizes of 100-150 Pd particles on several images taken from different places.
ACKKNOWLEDGMENTS
- This project was supported by funding from the Agency for Science, Technology and Research (A*STAR), Singapore.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML