-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2012; 2(6): 116-122
doi: 10.5923/j.pc.20120206.06
A First-Derivative Spectrophotometric Method for the Determination of Ciprofloxacin Hydrochloride in Ophthalmic Solution
Edith C. L. Cazedey1, Rudy Bonfilio2, Magali B. Araújo2, Hérida R. N. Salgado1
1Department of Drugs and Medicines, School of Pharmaceutical Sciences, University of São Paulo State, Araraquara, 14801-902, Brazil
2Department of Drugs and Medicines, School of Pharmaceutical Sciences, Federal University of Alfenas, Alfenas, 37130-000, Brazil
Correspondence to: Edith C. L. Cazedey, Department of Drugs and Medicines, School of Pharmaceutical Sciences, University of São Paulo State, Araraquara, 14801-902, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A sensitive, economical and reproducible derivative visible-spectrophotometric method for determination of ciprofloxacin hydrochloride in ophthalmic solution was developed and validated. The method is based on the development of yellow colored product due the reaction between ciprofloxacin hydrochloride and Fe (III). After the reaction, the spectra of the solutions were recorded in the range of 360-500 nm and the first-derivative spectra were obtained by instrumental electronic differentiation using a delta lambda (Δλ) of 8 nm. Analytical responses were units of distance from the central zero base line to the negative peak obtained at 386.4 nm. The method was completely validated according to the ICH guidelines, showing accuracy, precision, selectivity, robustness and linearity. The proposed method is simple, of low cost and provides reliable results in order to be used in quality control of ophthalmic solutions containing ciprofloxacin hydrochloride as active substance.
Keywords: Ciprofloxacin Hydrochloride, First-Derivative Spectrophotometry, Ophthalmic Solution, Pharmaceutical Analysis, Quinolones
Cite this paper: Edith C. L. Cazedey, Rudy Bonfilio, Magali B. Araújo, Hérida R. N. Salgado, "A First-Derivative Spectrophotometric Method for the Determination of Ciprofloxacin Hydrochloride in Ophthalmic Solution", Physical Chemistry, Vol. 2 No. 6, 2012, pp. 116-122. doi: 10.5923/j.pc.20120206.06.
Article Outline
1. Introduction
- Ciprofloxacin[1 – cyclopropyl – 6 - fluoro - 1,4 – dihydro – 4 – oxo – 7 - (1-piperazinyl) – 3 - quinolone carboxylic acid] (Figure 1) is a second generation fluoroquinolone antimicrobial agent with a wide spectrum of activity against Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa[1]. It is believed that the mode of action of fluoroquinolones is through binding DNA-gyrase enzyme[2].There are several analytical methods described in the literature for the determination of ciprofloxacinhydrochloride based on high performance liquid chromatography (HPLC) techniques[3-7]. HPLC is described as the official method for analysis of ciprofloxacin in the United States Pharmacopoeia, in the British Pharmacopoeia and in the Brazilian Pharmacopoeia[8-10]. However, HPLC techniques for routine analysis are often time consuming and expensive.For this reason, spectrophotometric techniques continues to be useful alternatives for the assay of different classes of drugs in pharmaceutical formulations and in biological samples due to its simplicity, speed, precision, accuracy,reasonable sensibility, and significant economicaladvantages over HPLC methods.Numerous methods using visible spectrophotometry [11-41] or UV spectrophotometry[42-44] have been reported for the determination of ciprofloxacin. However, these methods require expensive reagents, involve tedious extraction steps or need time for the reaction between the analyte and the colorimetric reagent.
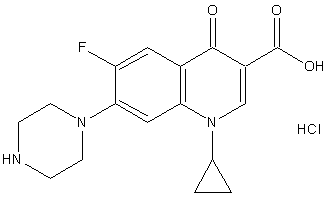 | Figure 1. Chemical structure of ciprofloxacin hydrochloride |
2. Experimental
2.1. Apparatus
- A Shimadzu UV-1601 PC, UV-visible, double beam spectrophotometer with matched quartz cells with path-length of 10 mm (Shimadzu Corp., Kyoto, Japan) was used and data were processed with online UVPC version 3.1 personal spectroscopy software (Shimadzu Corp., Kyoto, Japan). Chromatographic separations were carried out using a Waters Alliance® 2690 liquid chromatograph (Milford, USA) equipped with a Waters® high pressure 1525 pump binary grade, Waters® 2487 UV detector and 7725i manual injector with a 20 μL loop. The separation was performed on a Waters Symmetry® C–18 column (4.6 mm × 250 mm, 5.0 μm, Waters®, Milford, USA).
2.2. Chemicals and Reagents
- Ciprofloxacin hydrochloride reference substance was kindly provided by EMS Sigma Pharma Group (São Paulo, Brazil) and pharmaceutical forms were purchased in the Brazilian market. Methanol, acetic acid and acetonitrile were HPLC-grade and obtained from Sigma-Aldrich® (St. Louis, USA). Ophthalmic solutions were claimed to contain 3.5 mg mL-1 of drug and boric acid, sodium citrate, dissodium edetate (EDTA), benzalconium chloride and purity water as excipients. Ferric (III) chloride (FeCl2·6H2O) 1.0% aqueous solution was acquired from VETEC (Rio de Janeiro, Rio de Janeiro, Brazil). Milli-Q water was prepared by using a Millipore Milli-Q® UFPlus apparatus (Millipore, Milford, MA, USA) and meets USA Pharmacopoeia requirements.
2.3. Spectrophotometric Measurements
- Spectra of reference and sample solutions were recorded in the range of 360-500 nm at a medium scan speed (370 nm min-1) with a sampling interval of 0.2 nm and a fixed slit width of 2 nm. The first-derivative spectra were obtained by instrumental electronic differentiation (UVPC version 3.9 software) using a delta lambda (Δλ) of 8 nm. The amplitude values obtained in the first derivative spectra were arbitrary units of the distance from the central zero base line to the negative peak obtained at 386.4 nm. The spectrophotometric measurements were recorded by using Milli-Q water as a blank solution. All measurements were performed at ambient temperature.
2.4. Chromatographic Conditions
- In order to compare the results of the proposed derivative spectrophotometric method with a reference method, the same product batches were analyzed by a HPLC technique, which was developed and validated by our research group7. The mobile phase consisted of 2.5% acetic acid solution: methanol: acetonitrile (70:15:15, v/v/v). A flow rate of 1.0 mL min-1 was maintained. Injection volume was set to 20 μL. UV detection of the analyte was carried out at 275 nm.
2.5. Preparation of Solutions
2.5.1. Stock and Working Standard Solutions
- Stock standard solution containing 1000 µg mL-1 of ciprofloxacin hydrochloride was prepared in water. Working standard solutions were prepared by suitable dilutions of the corresponding stock solution into 10 mL volumetric flasks. To each flask, 1 mL of ferric chloride 1.0% aqueous solution was added and brought up to the volume with water. The analytical response of each solution was obtained according to section 2.3.
2.5.2. Sample Solutions
- Aliquots (200 µL) of ciprofloxacin hydrochloride ophthalmic solution (3500 µg mL-1) were transferred volumetrically into 10 mL volumetric flasks. To each flask, 1 mL of ferric chloride 1.0% aqueous solution was added and brought up to the volume with water to give a final concentration of 70.0 µg mL-1. The analytical response of each solution was obtained according to section 2.3.
2.6. Stability of Ciprofloxacin Hydrochloride Solution
- Ciprofloxacin hydrochloride standard solution at 50.0 µg mL-1 was analyzed at 0, 15, 30, 45 and 60 minutes after adding 1 mL of ferric chloride 1.0% aqueous solution.
2.7. Calibration Curve
- Calibration curve was constructed from the stock standard solution (1000 µg mL-1), by transferring aliquots of 0.5 to 1.0 mL of ciprofloxacin hydrochloride into 10 mL measuring flask, adding 1 mL ferric chloride 1.0% aqueous solution, and diluting to the mark with water. The solutions were measured in the amplitude of the peak against a blank prepared simultaneously.
2.8. Method Validation
- Method validation was performed following ICH specifications46 for selectivity, linearity, accuracy, precision, robustness, detection limit and quantitation limit.
2.8.1. Selectivity
2.8.2. Linearity
- Linearity was evaluated by regression analysis of ciprofloxacin hydrochloride solution at six concentration points, in triplicate, ranging from 50 to 100 µg mL-1 prepared on three consecutive days (n = 3). The values are reported as the mean ± S.D. of the calibration curves. Evaluation parameters, such as slope, intercept, correlation coefficient and squares residual sum, were calculated and presented.
2.8.3. Precision Assays
- Precision was evaluated with respect to both intraday and interday precision. Standard solutions were analyzed six times within the same day to obtain the intraday precision and six times over different days to obtain the interday precision. Each assay was carried out on a different sample of ciprofloxacin hydrochloride. The percentage relative standard deviation (RSD%) of the data obtained was calculated.
2.8.4. Accuracy
- To demonstrate the accuracy of the proposed method recovery studies were employed. This was carried out by adding known amounts of ciprofloxacin hydrochloride standard solution to the samples at three levels. All solutions were prepared in triplicate and analyzed. The percentage recovery was calculated.
2.8.5. Limit of Detection and Limit of Quantitation
- The LOD and LOQ were calculated by the formula LOD= 3.3(SD/a), LOQ= 10(SD/a), were SD is standard deviation of the 3.3 or 10 spectrophotometric readings of blank and a is a slope of calibration curves obtained in the linearity study.
2.8.6. Robustness
- Robustness was performed as a measure of the capacity of the analytical procedure to remain unaffected by small but deliberate variations in method performance parameters. The operational parameter investigated was the application of the method by different analysts. For this purpose, standard solutions were analyzed six times by two different analysts. The RSD% of the obtained data was calculated.
2.9. Application of the Validated Method to Pharmaceutical Products
- The applicability of the validated method was tested by analyzing ciprofloxacin hydrochloride in pharmaceutical dosage form. Moreover, the same product batches were analyzed by a HPLC technique[7]. The results were obtained by comparison of the sample spectrophotometric measured (n = 6) with those obtained from HPLC method (n = 6) at the same concentration level.
3. Results and Discussion
- Our research group has been developing analyticalmethods for some antibiotics based on visible spectrophotometry techniques[46-47]. Meantime, the determination of ciprofloxacin in ophthalmic solutions is more difficult due presence of excipients what can form complex with the reagents.In this work, some colorimetric reagents were tested and the addition of ferric chloride to ciprofloxacin hydrochloride in ophthalmic solutions resulted in a yellow colored product. After, experiments were performed to determine the optimum concentration and volume of the reagent used in the estimation of ciprofloxacin hydrochloride. The maximum absorbance was obtained with 1 mL of ferric chloride 1.0% aqueous solution; above this volume the absorbance remained unchanged. Therefore, 1 mL of this reagent was used in all further measurements.The reaction between iron (III) and ciprofloxacin is a fast reaction, in which Fe3+ react with the active carboxylic acid and the adjacent keto group in the 3- and 4- position, respectively, thus forming a six-membered ring[16]. In this reaction, the yellow coloration reaches maximum intensity immediately at room temperature and remains stables for at least 60 min.The zero-order UV spectrum of Fe(III)-ciprofloxacin complex showed a maximum drug absorption wavelength over 430 nm. However, significant interference from the colirium excipients was verified in the same region, which precludes the analytical use of zero-order spectrophotometry (Figure 2).For this reason, the first-order derivativespectrophotometric method (D1) was considered to be ideal for solving the overlapping of excipients absorption over ciprofloxacin hydrochloride signal. As observed in Figure 3, a zero-crossing for excipients appears at 386.4 nm. Therefore, this value was selected as optimum to determine ciprofloxacin hydrochloride in the presence of the pharmaceutical excipients, which are contained in ophthalmic solution. The second, third, and fourth derivatives were discarded because they showed insufficient selectivity and did not present analytical advantages.To verify the optimum Δλ for obtaining second-derivative spectra various values of Δλ were tested and the value of 8 nm was chosen as the most appropriate in order to give an adequate signal-to-noise ratio. Increasing Δλ, the signal-to-noise ratio improves and the fluctuation in a derivative spectrum decreases. However, if the value of Δλ is too large, the spectral intensity signal of second-derivative deteriorates.
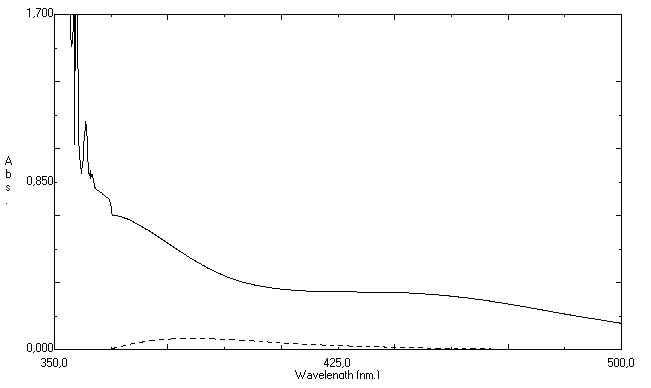 | Figure 2. Zero order absorption spectra of placebo ophthalmic solution (--) and standard ciprofloxacin hydrochloride 50.0 µg mL-1 (−) |
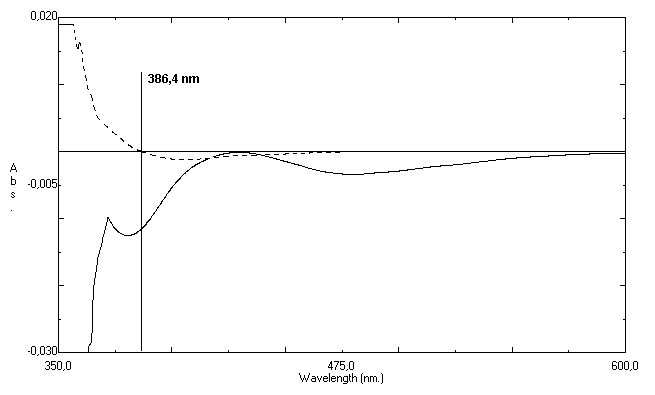 | Figure 3. First order derivative spectra of placebo ophthalmic solution (--) and standard ciprofloxacin hydrochloride at 50.0 µg mL-1 (−) |
3.1. Stability of Fe(III) Complex of Ciprofloxacin Hydrochloride in Aqueous Solution
- The results from stability study indicated that the behavior of the Fe(III)-ciprofloxacin complex in aqueous solution remained stable at room temperature up to 1 hour from its preparation.
3.2. Method Validation
- After method development, analytical validation was performed following ICH recommendations[45].
3.2.1. Selectivity
- The first-derivative spectra analyses show that formulation excipients of the ophthalmic solution did not interfere in the second-derivative spectrophotometric method (Fig. 3).
3.2.2. Linearity
- Using regression analysis, the following equation was obtained for the standard calibration curve of ciprofloxacin hydrochloride: D1 = -0.0002x -0.0009; where, D1 is the value of the first derivative signal at 386.4 nm of ciprofloxacin hydrochloride reacting with ferric chloride 1.0% aqueous solution and x is the concentration of ciprofloxacin hydrochloride (µg mL-1). The method was linear in the range of 50.0 to 100.0 µg mL-1 (r=0.9999). The calibration curve was in agreement with Beer’s law.
3.2.3. Precision
- Intraday and interday precision of the analytical method was found to be reliable based on RSD% (<2%). The values of 1.15% (n=6) and 1.34% (n=6) for intraday and interday precision, respectively, confirming that the method is sufficiently precise.
3.2.4. Accuracy
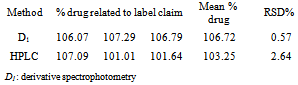
- The accuracy of the method was confirmed by determining the average of recoveries from the samples through the method of standard addition. As shown in Table 1, the percentage of recoveries at each level were in accordance with fixed limits of 98.0 to 102.0%, indicating the suitability of the developed method in quantifying the concentration of ciprofloxacin hydrochloride in ophthalmic solutions.
|
3.2.5. Limit of Detection and Limit of Quantitation
- Limit of detection and quantitation values were found to be, respectively, 0.18 and 0.60 mg L–1. These results demonstrated the sensitivity of the method.
3.2.6. Robustness
- The robustness of the method was determined by comparing the results obtained by two different analysts. The percentage of RSD, 2.92% (n = 12), between the analytical responses obtained by each analyst confirmed the robustness of the method, since the obtained values were within the acceptance limits (RSD% < 5.0%).
3.3. Application of the Validated Method to Pharmaceutical Products
- The proposed analytical method was applied for determination of ciprofloxacin hydrochloride in ophthalmic solutions. The results, expressed as percentage of drug related to label claim, are shown in Table 2. Moreover, the same product batches were analyzed by a HPLC technique and a t-test at a 0.05 significance level was applied to determine whether there is a statistically significant difference between the mean assay values of two proposed methods (Table 2). The statistical analysis revealed that there was no significant difference between the proposed method and an HPLC method for the determination of ciprofloxacin hydrochloride in ophthalmic solution (P>0.05).
|
4. Conclusions
- The proposed UV derivative spectrophotometric method proved to be an excellent option for ciprofloxacin hydrochloride determination in ophthalmic solutions. The interference from excipients was eliminated by selecting the most adequate wavelength. Furthermore, the proposed method does not require an elaborate treatment. The first derivative method was successfully applied for quantitative determination of ciprofloxacin hydrochloride and it can be used for analysis in routine quality control ofpharmaceuticals preparations with ciprofloxacin hydrochloride as a unique active substance. The method is simple, rapid, precise, selective, accurate, and of low cost.
ACKNOWLEDGEMENTS
- The authors thank CAPES (Brasília, Brazil),FUNDUNESP and PADC (Araraquara, Brazil) for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML