-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2012; 2(3): 37-42
doi: 10.5923/j.pc.20120203.03
Comparative Study of Tulsion A-23 and Indion -810 Strongly Basic Anion Exchange Resins By Application of 131I as a Tracer Isotope
Pravin U. Singare
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400 058, India
Correspondence to: Pravin U. Singare, Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400 058, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present study is an interesting application of 131I radioactive tracer isotope in characterization of nuclear and non-nuclear grade ion exchange resins Tulsion A-23 and Indion -810. The resins were characterized based on the specific reaction rate (min-1), percentage and amount of iodide ion exchange (mmol) and distribution coefficient values, under identical operating conditions. It is observed that using both the resins, there exist a strong positive correlation between amount of iodide ion exchanged and concentration of iodide ion solution; while a strong negative correlation exist between amount of iodide ion exchanged and temperature of iodide ion solution. The overall results indicate the superior performance of Tulsion A-23 over Indion -810 resins. It is expected that the technique used in the present investigation can be successfully extended to access the performance of different industrial grade ion exchange resins.
Keywords: Anion Exchange Resins, Radiotracer Isotopes, Tulsion A-23, Indion -810, Characterization
Article Outline
1. Introduction
- Though radioisotopes have been applied to the solution of problems in industry for over 50 years, research and development of the technology continues unabated. There are two main reasons for the continuing interest. Firstly, it is industry driven. Because of their unique properties, radioactive isotopes can be used to obtain information about plants and processes that cannot be obtained in any other way. Often, the information is obtained with the plant on-stream and without disrupting the process in any way. This can lead to substantial economic benefits, from shutdown avoidance to process optimization. Secondly, the methodology is derived from many fields of science and technology including radioisotope production, radiation detection, data acquisition, treatment and analysis, and mathematical modelling. The fundamental principle in radiochemical investigations is that the chemical properties of a radioisotope of an element are almost the same as those of the other stable/radioactive isotopes of the element. When radioisotope is present in a chemical form identical to that of the bulk of the element in a chemical process, then any reaction the element undergoes can be directly traced by monitoring the radioisotope. Radioisotope can also be tagged to a molecule or material to follow a process. Radiochemical work involves two main steps first is the sampling of chemical species to be studied and second is quantitative determination of the radiation emitted by the radioisotope in the sample [1]. Radiotracer methodology is described extensively in the literature [2]. Applications of radiotracers in chemical research cover the studies of reaction mechanism, kinetics, exchange processes and analytical applications such as radiometric titrations, solubility product estimation, isotope dilution analysis and autoradiography.The present investigation is one of the interesting applications of radiotracer isotopes to assess the performance of nuclear and non-nuclear grade ion exchange resins Tulsion A-23 and Indion -810 under different experimental conditions like temperature and concentration of ionic species present in the external exchanging medium.
2. Experimental
2.1. Conditioning of Ion Exchange Resins
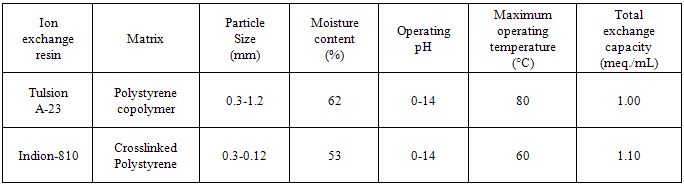
- Ion exchange resin Tulsion A-23 (by Thermax India Ltd., Pune), and Indion-810 (by Ion Exchange India Ltd., Mumbai) are strongly basic anion exchange resin in chloride and sulphite forms respectively having quaternary ammonium -N+R3 functional group. Details regarding the properties of the resins used are given in Table 1. These resins were converted separately in to iodide form by treatment with 10 % KI solution in a conditioning column which is adjusted at the flow rate as 1 mL / min. The resins were then washed with double distilled water, until the washings were free from iodide ions as tested by AgNO3 solution. These resins in iodide form were then dried separately over P2O5 in desiccators at room temperature.
|
2.2. Radioactive Tracer Isotope
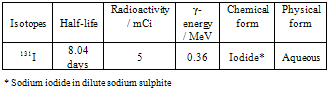
- The radioisotope 131I used in the present experimental work was obtained from Board of Radiation and Isotope Technology (BRIT), Mumbai. Details regarding the isotope used in the present experimental work are given in Table 2.
|
2.3. Study on Kinetics of Iodide Ion-Isotopic Exchange Reaction
- In a stoppered bottle 250 mL (V) of 0.001 M iodide ion solution was labeled with diluted 131I radioactive solution using a micro syringe, such that 1.0 mL of labeled solution has a radioactivity of around 15,000 cpm (counts per minute) when measured with γ -ray spectrometer having NaI (Tl) scintillation detector. Since only about 50–100 μL of the radioactive iodide ion solution was required for labeling the solution, its concentration will remain unchanged, which was further confirmed by potentiometer titration against AgNO3 solution. The above labeled solution of known initial activity (Ai) was kept in a thermostat adjusted to 30.00 °C. The swelled and conditioned dry ion exchange resins in iodide form weighing exactly 1.000 g (m) were transferred quickly into this labeled solution which was vigorously stirred by using mechanical stirrer and the activity in cpm of 1.0 mL of solution was measured. The solution was transferred back to the same bottle containing labeled solution after measuring activity. The iodide ion-isotopic exchange reaction can be represented as:
 | (1) |
|
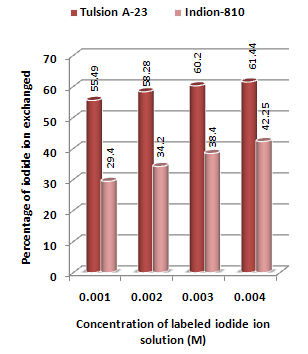 | Figure 2. Variation in Percentage Iodide Ions Exchanged with Concentration of Labeled Iodide Ion Solution Amount of ion exchange resin in iodide form = 1.000 g, Volume of labeled iodide ion solution = 250 mL, Temperature = 35.0 00C |
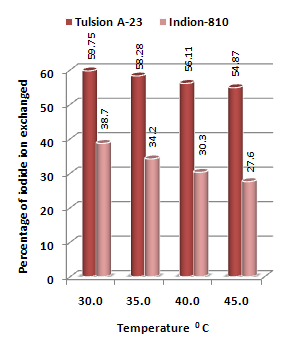 | Figure 3. Variation in Percentage Iodide Ion Exchanged with Temperature of Labeled Iodide Ion Solution Amount of ion exchange resin in iodide form = 1.000 g, Concentration of labeled exchangeable iodide ion solution = 0.002M, Volume of labeled iodide ion solution = 250 mL, Amount of exchangeable iodide ions in 250 mL labeled solution = 0.500 mmol |
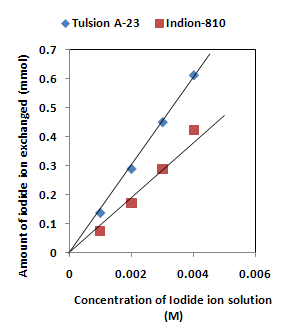 | Figure 4. Correlation between concentration of iodide ion solution and amount of iodide ion exchanged. Amount of ion exchange resin in iodide form = 1.000 g, Volume of labeled iodide ion solution = 250 mL, Temperature = 35.00 0C Correlation coefficient (r) for Indion-810 = 0.9973 Correlation coefficient (r) for Tulsion A-23 = 0.9999 |
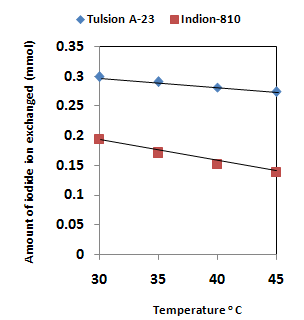 | Figure 5. Correlation between Temperature of exchanging medium and amount of iodide ion exchanged Amount of ion exchange resin in iodide form = 1.000 g, Concentration of labeled exchangeable iodide ion solution = 0.002M, Volume of labeled iodide ion solution = 250 mL, Amount of exchangeable iodide ions in 250 mL labeled solution = 0.500 mmol Correlation coefficient (r) for Indion-810 = -0.9942 Correlation coefficient (r) for Tulsion A-23 = -0.9979 |
3. Results and Discussion
3.1. Study of Iodide Ion-Isotopic Exchange Reactions
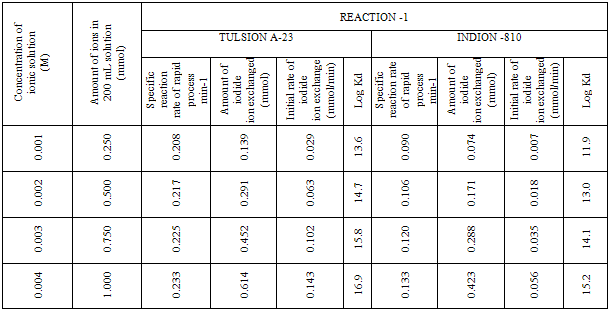
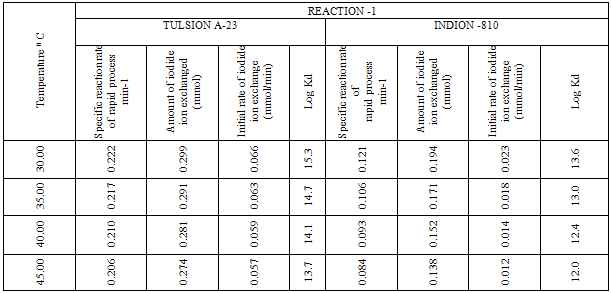
- In the present investigation it was observed that due to the rapid iodide ion-isotopic exchange reaction taking place, the activity of solution decreases rapidly initially, then due to the slow exchange the activity of the solution decreases slowly and finally remains nearly constant (Figure 1). Preliminary studies show that the above exchange reactions are of first order [10, 11]. Therefore logarithm of activity when plotted against time gives a composite curve in which the activity initially decreases sharply and thereafter very slowly, evidently rapid and slow ion-isotopic exchange reactions were occurring simultaneously(Figure 1)[4-9]. The specific reaction rate for the rapid exchange reaction was obtained by resolving the composite curve in the similar way as that explained in our previous work [4-9]. The amount of iodide ions exchanged (mmol) on the resin were obtained from the initial and final activity of solution and the amount of exchangeable ions in 250 mL of solution. From the amount of ions exchanged on the resin (mmol) and the specific reaction rates (min-1), the initial rate of ion exchanged (mmol/min) was calculated. For iodide ion-isotopic exchange reaction, at a constant temperature of 35.00 0C, the values of specific reaction rate increases from 0.208 to 0.233 min-1 for Tulsion A-23 and from 0.090 to 0.133 min-1 for Indion-810 when the concentration of iodide ion solution is increased from 0.001M to 0.004M (Table 3). However, when iodide ion concentration is kept constant (0.002M), the specific reaction rate was observed to decrease from 0.222 to 0.206 min-1 for Tulsion A-23 and from 0.121 to 0.084 min-1 for Indion-810 with rise in temperature from 30.00 °C to 45.00 °C (Table 4).From the knowledge of Ai, Af, volume of the exchangeable ionic solution (V) and mass of ion exchange resin (m), the Kd value was calculated by the equation
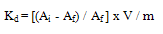 | (2) |
3.2. Comparative Study of Nuclear Grade Ion Exchange Resins
- From the Table 3, it is observed that for iodide ion-isotopic exchange reaction by using Tulsion A-23 resin, the values of specific reaction rate (min-1), amount of iodide ion exchanged (mmol), initial rate of iodide ion exchange (mmol/min) and log Kd were 0.208, 0.139, 0.029 and 13.6 respectively, which was higher than 0.090, 0.074, 0.007 and 11.9 respectively as that obtained by using Indion-810 resins under identical experimental conditions of 35.00 0C, 1.000 g of ion exchange resins and 0.001 M labeled iodide ion solution.It is observed that using Tulsion A-23 resins, at a constant temperature of 35.00 0C, as the concentration of labeled iodide ion solution increases 0.001 M to 0.004 M, the percentage of ions exchanged increases from 55.49 % to 61.44 %. While using Indion-810 resins under identical experimental conditions the percentage of ions exchanged increases from 29.40 % to 42.25 %. The effect of ionic concentration on percentage of ions exchanged is graphically represented in Figure 2. It is observed that using Tulsion A-23 resins, for 0.002 M labeled iodide ion solution, as the temperature increases 30.00 0C to 45.00 0C, the percentage of ions exchanged decreases from 59.75 % to 54.87 %. While using Indion-810 resins under identical experimental conditions the percentage of iodide ions exchanged decreases from 38.70 % to 27.60 %. The effect of temperature on percentage of ions exchanged is graphically represented in Figure 3.
3.3. Statistical Correlations
- The results of present investigation show a strong positive linear co-relationship between amount of iodide ions exchanged and concentration of iodide ion solution (Figure 4). In case of iodide ion exchange using Tulsion A-23 and Indion-810 resins, the values of correlation coefficient (r) was found to be 0.9999 and 0.9973 respectively. There also exist a strong negative co-relationship between amount of iodide ions exchanged and temperature of exchanging medium (Figure 5). For Tulsion A-23 and Indion-810 resins, the values of r were found to be -0.9979 and -0.9942 respective.The overall results indicate that under identical experimental conditions, as compared to Indion-810 resins, Tulsion A-23 resins shows higher percentage of iodide ions exchanged and hence show superior performance under operating conditions.
4. Conclusions
- The experimental work carried out in the present investigation will help to standardize the operational process parameters so as to improve the performance of selected nuclear grade ion exchange resins. The radioactive tracer technique used here can also be applied for characterization of different nuclear as well as non-nuclear grade ion exchange resins.
ACKNOWLEDGEMENTS
- The author is thankful to Professor Dr. R.S. Lokhande for his valuable help and support in carrying out the experimental work in Radiochemistry Laboratory of Department of Chemistry, University of Mumbai, Vidyanagari, Mumbai -58. The authors are extremely thankful to SAP Productions for developing and maintaining the manuscript template.
References
| [1] | Sood, D.D., Reddy, A.V.R., and N.Ramamoorthy, 2004, Applications of Radioisotopes In Physico-Chemical Investigations, in Fundamentals of Radiochemistry, Indian Association of Nuclear Chemists and Allied Scientists (IANCAS) Mumbai, India. |
| [2] | Radiotracer Applications in Industry- A Guidebook, Safety Reports, 2004, Series No. 423, International Atomic Energy Agency (IAEA) Vienna. |
| [3] | Sood, D.D., 1998, Proc., Int. Conf. on Applications of Radioisotopes and Radiation in Industrial Development, ed. Sood, D.D., Reddy, A.V.R., Iyer, S. R. K., Gangadharan, S., and Singh, G., B.A.R.C., Mumbai, India, 35–53. |
| [4] | Lokhande, R.S., Singare, P.U., and Kolte, A.R., 2010, Application of Radioactive Tracer Technique for Characterization of Strongly Basic Anion Exchange Resins Duolite A 101D and Duolite A 102D, Radiochemistry, 52(1), 81–86. |
| [5] | Singare, P.U., and Lokhande, R.S., 2009, Behaviour of Radioactive Iodide and Bromide ions from Aqueous Solution on Ion Exchange Resins Amberlite IRA-400, Natural Science, 1(1),191-194. |
| [6] | Lokhande, R.S., Singare, P.U., and Parab, S.A., 2008, Application of Radioactive Tracer Technique to Study the Kinetics of Iodide Ion- Isotopic Exchange Reaction using Strongly Basic Anion Exchange Resin Duolite A-116, Radiochemistry, 50(6), 642-644. |
| [7] | Lokhande, R.S., Singare, P.U., and Patil, V.V., 2008, Application of Radioactive Tracer Technique to Study the Kinetics and Mechanism of Reversible Ion- Isotopic Exchange Reaction using Strongly Basic Anion Exchange Resin Indion -850, Radiochemistry, 50(6), 638-641. |
| [8] | Lokhande, R. S., Singare, P. U., and Dole, M. H., 2007, Application of Radiotracer Technique to Study the Ion Isotope Exchange Reactions Using a Strongly Basic Anion-Exchange Resin Duolite A-113, Radiochemistry, 49(5), 519-522. |
| [9] | Lokhande, R. S., Singare, P. U., and Karthikeyan, P., 2007, The Kinetics and Mechanism of Bromide Ion Isotope Exchange Reaction in Strongly Basic Anion-Exchange Resin Duolite A-162 Determined by the Radioactive Tracer Technique, Russ. J. Physical Chemistry A, 81(11), 1768–1773. |
| [10] | Lokhande, R. S., and Singare, P. U., 2003, Study of reversible ion-isotopic self diffusion reaction using 82 Br as a radioactive tracer isotope, Asian J. Chem., 15(1), 33-37. |
| [11] | Lokhande, R. S., and Singare, P. U., 2005, Study on kinetics of self diffusion reaction by application of 82 Br as a radioactive tracer isotope, Asian J. Chem., 17(1), 125-128. |
| [12] | Heumann, K. G., and Baier, K., 1982, Chloride distribution coefficient on strongly basic anion-exchange resin: Dependence on co-ion in alkali fluoride solutions, Chromatographia, 15(11), 701-703. |
| [13] | Adachi, S., Mizuno, T., and Matsuno, R., 1995, Concentration dependence of the distribution coefficient of maltooligosaccharides on a cation-exchange resin, J. Chromatography A, 708(2), 177-183. |
| [14] | Shuji, A., Takcshi, M., and Ryuichi, M., 1996, Temperature dependence of the distribution coefficient of maltooligosaccharides on cation-exchange resin in Na+ form, Bioscience, Biotechnology, and Biochemistry, 60(2), 338-340. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML