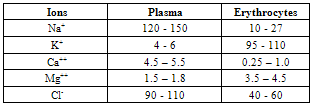
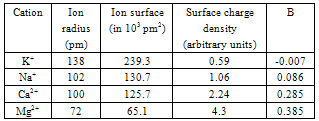
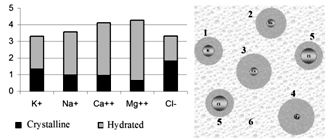
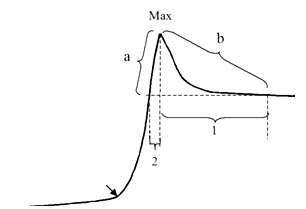
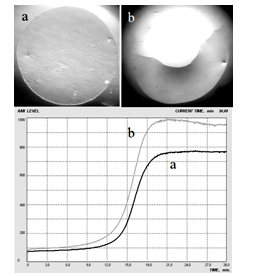
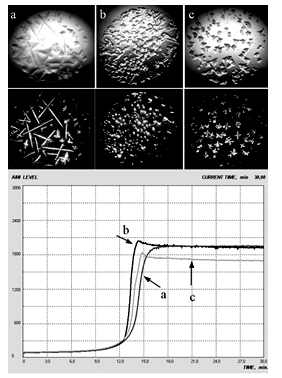
| [1] | A.Ya. Yaroshevsky (ed.), “The Physiology of Blood System”, Series “Handbook of Physiology”, Nauka: Leningrad, Russia, 1968[in Russian]. |
| [2] | F. Hofmeister, “On the understanding of the effect of salts”, Second report, “On regularities in the precipitation effects of salts and their relationship to their physiological behavior”, Archiv Exp. Pathol. Pharmakol. (Leipzig), vol. XXIV, pp.247-60, 1887. |
| [3] | P. Debye, E. Hückel, “On the theory of electrolytes. 1. Freezing point depression and related phenomena”, Phys. Z., vol.24, pp.185-206, 1923. |
| [4] | G. Jones, M. Dole, “The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride”, J. Am. Chem. Soc., vol.51, pp.2950-2964, 1929. |
| [5] | E.R. Nightigale, “Phenomenological theory of ion solvatation. Effective radii of hydrated ions”. J. Phys. Chem., vol.3, no.5, pp.1381-1387, 1959. |
| [6] | N.T. Skipper, G.W. Neilson, “X-ray and neutron-diffraction studies on concentrated aqueous solutions of sodium-nitrate and silver-nitrate”, J. Phys. Condens. Matter., vol.1, pp.4141-4154, 1989, |
| [7] | K.D. Collins, “Charge density-dependent strength of hydration and biological structure”, Biophys. J., vol.72, pp.65-76, 1997. |
| [8] | M.Y. Kiriukhin, K.D. Collins, “Dynamic hydration numbers for biologically important ions”, Biophys. Chem., vol.99, pp.155-168, 2002. |
| [9] | W. Kunz, P. Lo Nostro, B.W. Ninham, “The present state of affairs with Hofmeister effects”. Curr. Opin. Colloid Interface Sci., vol.9, no.1-2, pp.1-18, 2004. |
| [10] | K.D. Collins, G.W. Neilson, J.E. Enderby, “Ions in water: Characterizing the forces that control chemical processes and biological structure”, Biophys. Chem., vol.128, pp.95-104, 2007. |
| [11] | Y. Kohno and H. Ohno, “Temperature-responsive ionic liquid / water interfaces: relation between hydrophilicity of ions and dynamic phase change”, Phys. Chem. Chem. Phys., vol. 14, pp.5063-5070, 2012. |
| [12] | F. Bruni, S. Imberti, R. Mancinelly and M.A. Ricci, “Aqueous solutions of divalent chlorides: ion hydration shell and water structure”, J. Chem. Phys., vol.136, pp. 064512 (7 pages), 2012. |
| [13] | J. E. Gordon, “The Organic Chemistry of Electrolyte Solutions”, Wiley: New York, USA, 1975. |
| [14] | I.B. Ivanov, R.I. Slavchov, E.S. Basheva, D. Sidzhakova, S.I. Karakashev, “Hofmeister effect on micellization, thin films and emulsion stability”, Adv. Colloid Interface Sci, vol.168, pp.93-104, 2011. |
| [15] | C.W. Bock, A. Kaufman, J.P. Glusker, “Coordination of water to magnesium cations”, Inorg. Chem., vol.33, pp.419-427, 1994. |
| [16] | Z.K. Blinnikova, K.V. Mayerle, M.P. Tsuryupa, V.A. Davankov, “Characteristic features of mineral salt separation by thе method of frontal exclusion chromatography on neutral nanoporous supercross-linked polystyrene”, Sorption and Cromatographic Processes, vol.9, no.3, pp.323-331, 2009[in Russian]. |
| [17] | G. Archontis, E. Leontidis, G. Andreou, “Attraction of ions by the free water surface, revealed by simulations with a polarizable force field based on drude oscillators”, J. Phys. Chem. B, vol.109, pp.17957-17966, 2005. |
| [18] | M. Shibukawa, Y. Kondo, Y. Ogiyama, K. Osuga, Sh. Saito, “Interfacial water on hydrophobic surfaces recognized by ions and molecules”, Phys. Chem. Chem. Phys., vol 13, pp. 15925-15935, 2011. |
| [19] | P. Jungwirth, D.J. Tobias, “Specific ion effects at the air/water interface”, Chem. Rev., vol.106, pp.1259-1281, 2006. |
| [20] | P.B. Petersen, R.J. Saycally, “On the nature of ions at the liquid water surface”, Annu. Rev. Chem., vol.57, pp.333-64, 2006. |
| [21] | Y. Levin, “Polarizable ions at interfaces”, Phys. Rev. Lett., vol. 102, pp. 147803, 2009. |
| [22] | Y. Levin, A.P. dos Santos, A. Diehl, “Ions at the air-water interface: an end to the hundred-year-old mystery?”, Phys. Rev. Lett., vol. 103, pp. 257802, 2009. |
| [23] | M. Chaplin, “Theory and experiment: what is the surface charge of water?” Water, vol.1, pp.1-28, 2009. |
| [24] | R. Vaґcha, M.L. Berkowitz, P. Jungwirth, “Molecular model of a cell plasma membrane with an asymmetric multicomponent composition: Water permeation and ion effects”, Biophys. J., vol.96, pp.4493–4501, 2009. |
| [25] | L.Vrbka, J. Vondrasek, B. Jagoda-Cwiklik, R. Varcha, P. Jungwirth, “Quantification and rationalization of the higher affinity of sodium over potassium to protein surfaces”, PNAS, vol.103, no.42, pp.15440-15444, 2006.www.pnas.org_cgi_doi_10.1073_pnas.0606959103 |
| [26] | C. Caleman, J. S. Hub, P. J. Van Maaren, D. Van der Spoel, “Atomistic simulation of ion solvation in water explains surface preference of halides”, PNAS, vol.108, pp.6838-6842, 2011. Www.pnas.org/cgi/doi/10.1073/pnas.1017903108 |
| [27] | V. Buch, A. Milet, R. Varcha, P. Jungwirth, J.P. Devlin, “Water surface is acidic”, PNAS, vol.104, no.18, pp.7342-7347, 2007.www.pnas.org_cgi_doi_10.1073_pnas.0611285104 |
| [28] | C. Colacicco, “Electrical potential of the water surface”, Chemica Scripta, vol.28, no.2, pp.141-144, 1988. |
| [29] | G. Lamoureux, R. Saycally, “Absolute hydration free energy scale for alkali and halide ions established from simulations with a polarizable force field”, J. Phys. Chem. B, vol.110, pp.3308-3322, 2006. |
| [30] | Ya.E. Geguzin, “The Drop”, Nauka: Moscow, Russia, 1973[in Russian]. |
| [31] | R D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S.R. Nagel, T.A. Witten, “Contact line deposits in an evaporating drop”, Phys. Rev. E, vol.62, no.1, pp.756-765. 2000. |
| [32] | R. D. Deegan, “Pattern formation in drying drops”, Phys. Rev. E, vol.61, no.1, pp.475-485. 2000. |
| [33] | Yu. Popov, “Evaporative deposition patterns: Spatial dimensions of the deposit”, Phys. Rev. E, vol.71, pp.036313, 2005. |
| [34] | L. Pauchard, “Patterns caused by buckle-driven delamination in desiccated colloidal gels”, Europhys. Lett., vol.74, no.1, pp.188-192, 2006. |
| [35] | Yu. Yu. Tarasevich, I.V. Vodolazskaya and O. P. Isakova, “Desiccating colloidal sessile drop: dynamics of shape and concentration”, Colloid and Polymer Science, vol. 289, no 9, pp.1015-1023, 2011. |
| [36] | D. Sobac and D. Brutin, “Structural and evaporative evolutions in desiccating sessile drops of blood”, Phys. Rev. E., vol. 84, pp. 011603, 2011. |
| [37] | D. Sobac and D. Brutin, “Triple-line behavior and wettability controlled by nanocoated substrates: influence on sessile drop evaporation”, Langmiur, vol. 27, no 24, pp. 14999-15007, 2011. |
| [38] | D. Sobac and D. Brutin, “Thermocapillary instabilities in an evaporating drop deposited onto a heated substrate”, Phys. Fluids, vol. 24, pp. 032103, 2012. |
| [39] | T.A.H. Nguen, A.V. Nguen, M.A. Hampton, Z.P. Xu, L. Huang, V. Rudolf, “Theoretical and experimental analysis of droplet evaporation on solid surfaces”, Chemical Engineering Science, vol. 69, no 1, pp. 522-529, 2012. |
| [40] | Т.А. Yakhno, V.G. Yakhno, “Structural evolution of drying drops of biological fluids”, Technical Physics, vol.54, no.8, pp.1219-1227, 2009. |
| [41] | T. Yakhno, “Salt-induced protein phase transitions in drying drops”, J. Colloid Interface Sci., vol.318, pp.225-230, 2008. |
| [42] | V. Ragoonanan, A. Aksan, “Heterogenity in desiccated solutions: implications for biostabilization”, Biophys. J., vol.94, pp.2212-2227, 2008. |
| [43] | E.G. Rapis, “Regular structure formation of drying protein film”, Pis’ma v JTF, vol.14, no.17, pp.1560-1564, 1988[in Russian]. |
| [44] | Yu. Yu. Tarasevich, A. K. Ayupova, “Effect of diffusion on the separation of components in a biological fluid upon wedge-shaped dehydration”, Technical Physics, vol.48, no.5, pp.535–540, 2003. |
| [45] | T. A. Yakhno, V. V. Kazakov, O. A. Sanina, A. G. Sanin, V. G. Yakhno, “Drops of biological fluids drying on a hard substrate: Variation of the morphology, weight, temperature, and mechanical properties”, Technical Physics, vol.55, no.7, pp.929-935, 2010. |
| [46] | L.V. Savina, “Crystalloscopic Structures of Blood Serum of a Healthy and Diseased Persons”, Sovetskaya Kuban: Krasnodar, Russia, 1999[in Russian]. |
| [47] | V.N. Shabalin, S.N. Shatokhina, “Morphology of Biological Liquids of a Human”, Khrizostom: Moscow, Russia, 2001[in Russian]. |
| [48] | H. Y. Erbil, “Evaporation of pure liquid sessile and spherical suspended drops: a review”, Advances in Colloid and Interface Science, vol. 170, pp. 67-86, 2012. |
| [49] | T.A. Yakhno, V.G. Yakhno, A.G. Sanin, I.I. Shmelev, “Study of the dynamics of phase transitions of liquids of different types by recording acoustomechanical impedance of a drying drop”. Biofizika, vol.47, no.6, pp.1101-1105, 2002[in Russian]. |
| [50] | T.A. Yakhno, V.G. Yakhno, A.G. Sanin, O.A. Sanina, A.S. Pelyushenko, “Dynamics of phase transitions in drying drops as an information parameter of liquid structure”, Nonlinear Dynamics, vol.39, pp.369-374, 2005. |
| [51] | T. Yakhno, A. Sanin, A. Pelyushenko, V. Kazakov, O. Shaposhnikova, A. Chernov, V. Yakhno, C. Vacca, F. Falcone, B. Johnson, “Uncoated quartz resonator as a universal biosensor”, Biosensors & Bioelectronics, vol.22, no.9-10, pp.2127-2131, 2007. |
| [52] | T. A. Yakhno, A. G. Sanin, C. V. Vacca, F. Falcione, O. A. Sanina, V. V. Kazakov, V. G. Yakhno, “A New Technology for Studying Multicomponent Liquids Using a Quartz Crystal Resonator: Theory and Applications”, Technical Physics, vol.54, no.10, pp.1423–1430, 2009. |
| [53] | T. Yakhno, A. Sanin, V. Kazakov, O. Sanina, C. Vacca, F. Falcione, V. Yakhno, “Uncoated quartz resonator as a universal biosensor”, Chapter in a book: “Intelligent and Biosensors”, Vernon, S. (ed.) Somerset: INTECH, Austria, pp.386-404, 2010.Http://www.sciyo.com/articles/show/title/uncoated-quartz-resonator-as-a-universal-biosensor |
| [54] | T. Yakhno, “Blood as a Polydisperse System”, Lambert Academic Publishing gmbh & Co. KG, Saarbrucken, Germany, 2011. |
| [55] | T.A.Yakhno, V.V. Kazakov, A.G. Sanin, O.B. Shaposhnikova, A.S. Chernov, “Dynamics of phase transitions in drying drops of human serum protein solutions”, Technical Physics, vol.52, no.4, pp.515–520, 2007. |
| [56] | A.N. Kirgintsev, L.N. Trushnikova, V.G. Lavrentieva, “Water Solubility of Inorganic Substances”, Handboook, Chemistry: Leningrad, Russia, 1972[in Russian]. |
| [57] | M.I. Gelfman, O.V. Kovalevich, V.P. Yustratov, Colloid Chemistry, 2nd edition; Lan: Moscow, Russia, 2004[in Russian]. |
| [58] | I.K. Kikoin (ed), “Tables of Physical Quantities”, Atomizdat: Moscow, Russia, 1976[in Russian]. |
| [59] | H. Hu, R.G. Larson, “Analysis of the Effects of Marangoni Stresses on the Microflow in an Evaporating Sessile Droplet”, Langmuir, vol.21, no. 9, pp.3972-3980, 2005. |
| [60] | W.D. Ristenpart, P.G. Kim, C. Domingues, J. Wan, H.A. Stone, “Influence of substrate conductivity on circulation reversal in evaporating drops”. Phys. Rev. Lett., vol.99, pp.234502, 2007. |
| [61] | R. Bhardwaj, X. Fang, D. Attinger, “Pattern formation during the evaporation of a colloidal nanoliter drop: a numerical and experimental study”, New J. Phys., vol.11, pp.075020, 2009. |

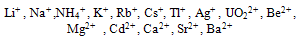
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML