-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2012; 2(1): 21-26
doi:10.5923/j.pc.20120201.05
Water Clusters in Liquid Fuels. Their Role and Surroundings
Kristina Zubow1, Anatolij Zubow2, Viktor Anatolievich Zubow1
1A IST Handels- und Consulting GmbH, dept. R&D, D-17192 Groß Gievitz, Germany
2Dept. of Computer Science, Humboldt University Berlin, Johann von Neumann Haus, D-12489 Berlin
Correspondence to: Viktor Anatolievich Zubow, A IST Handels- und Consulting GmbH, dept. R&D, D-17192 Groß Gievitz, Germany.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The long-range order in n-hexane, gasoline, diesel and in their mixtures with/without water is investigated by the gravitational mass spectroscopy (GMS). Molecular clusters are analyzed to be present in fuels and mixtures. Using GMS subtraction spectra for water in hydrocarbons, it becomes clear what role water plays and how it interact with the surroundings. Water in fuels is concluded to appear as individual clusters, whose structure (density) depends on the nature of hydrocarbon clusters. The combustion mechanism of hydrocarbons saturated with water will be discussed. Water clusters are suggested to accelerate the diffusion processes of the combustion. Molecular clusters in liquid fuels are formed in stationary gravitational waves of white noises, penetrating the Earth.
Keywords: Gasoline, Diesel, Clusters, Burning, Water, Diffusion, White noises
Cite this paper: Kristina Zubow, Anatolij Zubow, Viktor Anatolievich Zubow, Water Clusters in Liquid Fuels. Their Role and Surroundings, Physical Chemistry, Vol. 2 No. 1, 2012, pp. 21-26. doi: 10.5923/j.pc.20120201.05.
1. Introduction
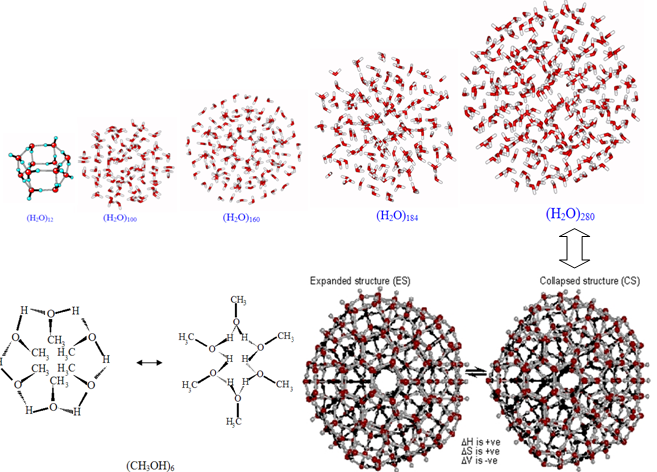
- The mixing of different liquids should not lead to the formation of homogeneous systems, the so-called ideal solutions because of cohesive and structural factors, the crucial role of which, unfortunately, is ignored by many researchers. If even simple liquids are considered to be inhomogeneous (long-range order at cluster level), then the mixtures of different miscible liquids even more. On the other hand, we can expect that the heating will lead to the dispersion of the mixture of liquids and the cohesive and structural factors coupled with the positive influence of white noise to the formation of a new long-range order. The mixtures are to be understood as nano colloidal systems where the nanoparticles of different liquids are not miscible with each other although a phase boundary can not be seen with the classical energy-rich methods. However, some simple water clusters (up to hexamers, 3329 cm-1) in neon were already recorded with IR spectroscopy by Hibarayashi and Yamada[1]. Nevertheless, a new non-destructive method for analyzing the long-range order in mixtures of liquids is necessary.Understanding of liquids’ states in mixtures will open new fields in nanotechnology, optimize known processes in chemistry (drying, combustion, polymerization), model biological systems, and develop the remote communication of liquids.In this work, we decided to study the long-range order in fuels, as the most promising topics.To get an idea on the long-range order in liquid fuels is of great economic importance in terms of optimizing combustion processes, dissolution, film formation, and even oil exploration. Scientific interest is dictated by the need to understand the formation mechanisms of clusters, their physical chemical properties as kinetically independent units, the cluster distribution in a liquid furthermore, to investigate cluster surfaces and their gravitational fields. The coming energy problems force to search for new ways to use oil rationally, especially when it will be burned. Combustion engines have reached their limit and new engineering ideas can not significantly reduce the fuel consumption. However, in recent years a number of patents has been applied concerning the possibility of reducing fuel consumption by adding water to it and dispersing the emulsion prior to injection into the cylinder combustion chamber[1-4]. Literature published on this topic is almost simultaneously researched. For instance, to Fu et al[1] gasolines investigated with gas chromatography are characterized by a cluster structure (up to 20 different clusters); different types of gasoline are even identified with the cluster structure. After passing through a porous ceramic membrane or treatment with electrical current the water-gasoline mixture forms “ultra disperse” clusters.Modeling the water cluster formation in n-hexane at 300 K Mudzhikova et al[1] found that three cluster types consisting of 27, 64 and 125 molecules are possible.The cluster formation energy is found to be really low[1-3] where the cluster stability is ensured by the entropy factor.Applying traditional methods of physical chemistry it is difficult to detect and identify clusters[1,9,1], however, computer simulations make it possible to calculate hypothetical structures with a minimum of energy.For a better understanding of the material in this work Figure 1 shows some modeled clusters of elementary liquids (see[1] too) and Figure 2 gives review GMS spectra of some liquid hydrocarbons.
2. Experimental Part
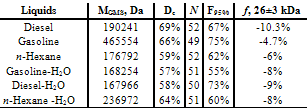
- As investigation objects n-hexane (nD20 = 1.3752), diesel (DIN EN 228), gasoline (ROZ DIN EN 98) and mixtures of liquids are used. The fuel mixtures are prepared at 293 K by simple mixing, the hydrocarbons’ saturation with distilled water by shaking and following decantation of the upper fraction. Samples are placed in a box being maximally protected from outer energy flows[13]. Measurement method is described in[8,12,13]. Before the measurement, the samples are stored for a week under exclusion of light and mechanical fields. The GMS sensor is directly placed inside the sample (10 ml). The negative signal in the GMS (-f) represents the energy release of a collapsed cluster leading to its higher density and crystallization. On the other side the positive signal (f) means the energy absorption at the interaction of the shock wave with the expanded cluster and its melting. The values of collapsed clusters are therefore described with minus and that of expanded one with plus. More information, see in[12]. MGMS – average molecular mass of all clusters in Dalton (Da), MGMS=Σ│f│·m), m – cluster mass (Da), f – energetical part of a cluster in ensemble of clusters, -f part of collapsed clusters, Dc – sum of all collapsed clusters and N – number of cluster kinds in ensemble, p – shock wave pressure.According to this Figure the GMS-spectra of hydrocarbons and their mixtures are similarly however, there are some significant differences. For example, the cluster of 20 molecules oscillates in n-hexane[8] (labeled with an arrow) as a collapsed cluster while it is shown as expanded one in gasoline.When 1 vol. % diesel is added to gasoline, then the GMS- spectrum changes dramatically. The strong change in the spectrum continues with further addition of diesel. At a diesel content of 10 to 30 vol. % the distribution of clusters and their interaction with surroundings approach to that one in pure n-hexane.In the literature there is not enough information about clusters formed by organic compounds[8,9,14]. Generally, the simplest molecule clusters, such as benzene dimers[15], complexes of water with acetone[15,16] and other organic compounds with polar groups[17] were studied.
 | Figure 2. Review GMS spectra of hydrocarbons. 286 K. Cluster ensembles up to 3 million Daltons, p > 1 N/m2. Diesel and its mixtures with gasoline, vol. % |
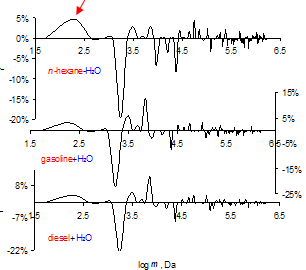 | Figure 3. Review GMS spectra of fuels saturated with water. 286 K. Mass ensembles up to 3 million Daltons, p> 1 N/m2 |
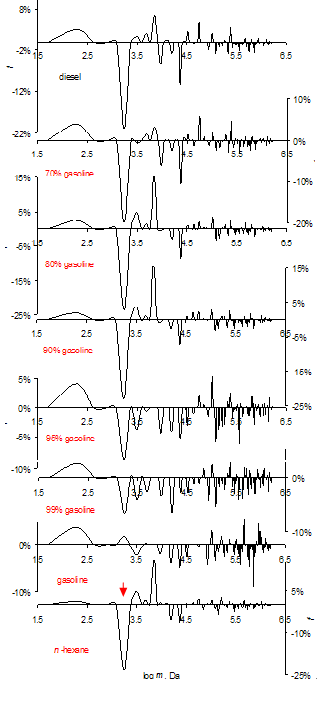
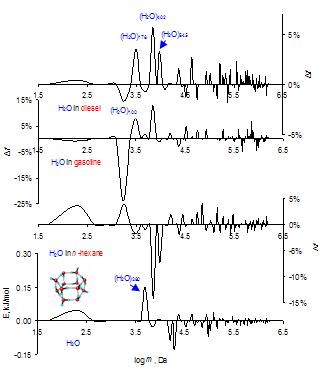 | Figure 4. GMS subtraction spectra for water in hydrocarbons (explanation in text). Mass ensembles up to 3 million Daltons, p> 1 N/m2 |
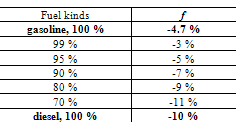
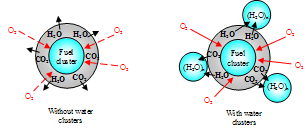 According to this scheme, the stream of reaction products prevent the penetration of oxygen into the fuel cluster however, the addition of water provides the possibility for the fast removal of the reaction products. In the following the combustion process will be accelerated, which leads to an increased fuel efficiency and higher engine power.In conclusion, we consider the behavior of the unique cluster with the mass of 26 ± 3 kDa analyzed in both liquids and polymeric materials[25]. In the present study, this cluster is observed to be present in water, hydrocarbons and their mixtures thereof. Both for the mixtures of water with hydrocarbons and water itself (GMS subtraction spectra for water in hydrocarbons according to the procedure given for the Figure 4), the interaction of this cluster with the surroundings remained almost constant at -8 %...- 9% (see also Table 1). The cluster of 26 kDa is also present in mixtures of hydrocarbons but its oscillation and interaction with the surroundings depend on the kind of fuel mixture. Table 2 presents the results obtained for this cluster.
According to this scheme, the stream of reaction products prevent the penetration of oxygen into the fuel cluster however, the addition of water provides the possibility for the fast removal of the reaction products. In the following the combustion process will be accelerated, which leads to an increased fuel efficiency and higher engine power.In conclusion, we consider the behavior of the unique cluster with the mass of 26 ± 3 kDa analyzed in both liquids and polymeric materials[25]. In the present study, this cluster is observed to be present in water, hydrocarbons and their mixtures thereof. Both for the mixtures of water with hydrocarbons and water itself (GMS subtraction spectra for water in hydrocarbons according to the procedure given for the Figure 4), the interaction of this cluster with the surroundings remained almost constant at -8 %...- 9% (see also Table 1). The cluster of 26 kDa is also present in mixtures of hydrocarbons but its oscillation and interaction with the surroundings depend on the kind of fuel mixture. Table 2 presents the results obtained for this cluster.
|
|
3. Conclusions
- At the level of molecular mass concentrations – clusters, liquid fuels are characterized by a long-range order they are designated as nano-colloidal systems, therefore.In fuel mixtures proceeds a distribution of molecules according to their nature and new cluster ensembles will be formed, which are stabilized by gravitational white noises.The role of water in the formation of long-range order in liquid fuels is that it favors the formation of a thermodynamically stable nanoemulsion.The hierarchy in the formation of nanoemulsions represented by molecules’ clusters of the same nature is a sequence of oscillating masses (clusters and their associates at submicellar and micellar level). The results obtained can be applied for a rapid and non-destructive monitoring of liquid fuels and other petroleum products.
References
| [1] | Shinichi Hirabayashi, Koichi MT Yamada. The monocyclic water hexamer detected in neon matrices by infrared spectroscop. Chem. Phys. Letters 2007;435; 74-8. |
| [2] | Urano Noritsugu. Functional water for improvement of hydrocarbon based liquid fuel JP 2001064662 А2 20010313. Application No. JP 1999-241435 1999 0827, IPC: C10L001-12 Jpn. Kokai Tokkyo Koho (2001), 5. |
| [3] | Uzawa Masakazu. Method for treating for hydrocarbon fuel JP 2002256273 A2 20020911, Applic. No. JP 2001-59782 20010305. Jpn. Kokai Tokkyo Koho (2002), 11 pp. IPC: C10G032-00. |
| [4] | McVitty Aaron. Improvement relating to fuel conditioning PCT Int. Appl. (2004) 19pp. WO 2004044101 A2 20040527. Appl. WO 2003- GB4955 20031114. |
| [5] | Johnson Keith H, Zhang Bin. Stabilized water nanocluster-fuel emulsion designed through quantum chemistry PCT Int. Appl. (1998), 91 pp. WO 9821294 A1 19980522. Appl.: WO 97- US20779 1997114. IPC: C10L001-32. |
| [6] | Fu da-you, Yuan Dong, Tan Wen-yuan, Xiang Shuang-guan. Cluster analysis method for gasoline.Jilin Huagong Xueyuan Xuebao 2005;22;6-10. |
| [7] | Mudzhikova GV, Brodskaya EN. Molecular dynamics simulation of small water clusters in nonpolar n-hexane. Vestnik Sankt Petersbugskogo universiteta, seria 4. Fisika, Chimija 2004;4;71-7, in Russian |
| [8] | Zubow KV, Zubow AV, Zubow VA. Cluster structure of liquid alcohols, water and n-Hexane. J. of Appl. Spectr 2005;72;321-28. |
| [9] | Zakharov VV, Brodskaya EN, Laaksonen A. Molecular dynamics simulation of methanol clusters. J. Chem. Phys 1998;109; 9487-9493. |
| [10] | Shruti Maheshwary, Nitin Patel, Narayanasami Sathyamurthy. Structure and Stability of Water Clusters (H2O) n, n=8−20: An Ab Initio Investigation. J. Phys. Chem. A 2001;105; 10525-37. |
| [11] | Fesenko E.E., Terpugov E.A. About unusual spectral properties of water in a thin layer. Biofisika 1999;44;5-9, in Russian. |
| [12] | Zubow KV, Zubow AV, Zubow VA. Principles of gravitation spectroscopy. New form of molecular matter. Processes. Fields. Aist Handels- und consulting gmbH, dep. R&D, Berlin, electronic book (www.zubow.de),; 2010. (in Russian). |
| [13] | Zubow KV, Zubow AV, Zubow VA. Ensemble of Clusters – New Form of Molecular Matter, Risks and Сhances. Zubow Equations. In: Taylor JC, editor. Advances in Chemistry Research, New York: Novapublisher; 2010, vol. 5, p. 107-45. |
| [14] | Pagliai M., Cardini G., Righini R., Schettino V. Hydrogen bond dynamics in liquid methanol. J. Chem. Phys. 2003;119; 6655-63. |
| [15] | Tomas Rocha-Rinza, Luca De Vico, Valera Veryazov, Björn O. Roos. A theoretical study of singlet low-energy excited states of the benzene dimer. Chem. Phys. Letters 2006; 426;268-72. |
| [16] | Herbert C. Georg, Kaline Continho, Sylvio Canuto. Converged electronic polarization of acetone in liquid water and the role in the n–π* transition. Chem. Phys. Letters 2006;429; 119-23. |
| [17] | Erin R. Johnson, Gino A. DiLabio. Structure and binding energies in van der Waals dimers Comparison between density functional theory and correlated ab initio methods. Chem. Phys. Letters 2006;419; 333-39 |
| [18] | Lenz A, Ojamäe L. On the stability of dence versus cage-shaped water clusters: Quantum- chemical investigations of zero-point energies, free energies, basis-set effects and IR spectra of (H2O)12 and (H2O)20. Chem. Phys. Letters 2006;418;361-67. |
| [19] | Yoshiteru Yonetani. A severe artifact in simulation of liquid water using a long cut-off length: Appearance of a strange layer structureChem. Phys. Letters 2005;406;49 - 53. |
| [20] | Giuseppe Graziano. Benzene solubility in water: A reassessment. Chem.Phys. Letters 2006;429;114 -18. |
| [21] | Upadhyay D.M., Mishra P.C. Binding of benzene with water clusters (H2O)n, n=1–6, in the ground and lowest singlet excited states. THEOCHEM 2002;584;113 - 33. |
| [22] | Keith M. Murdoch, Thomas D. Ferris, John C. Wright, Thomas C. Farrar. Infrared spectroscopy of ethanol clusters in ethanol–hexane binary solutions. J of Chem. Phys 2002; 116; 5717-25. |
| [23] | Dolgopolova AV, Kushnarev DF, Qua KIm En, Kalabin GA, Schmidt FK. Proton and oxigen- 17 NMR spectroscopic study of hidrophilic-hydrophobic interactions in aqueaus solutions of petrolium products. Khimiya I technologiya vody 1993;15;107-12, in Russian. |
| [24] | Bogdanov EV, Manturova GM. Equiclaster model of water. Biomedicinskaja Radioelektronika 2000;7;19-28, in Russian. |
| [25] | Zysman V, Nguyen Tuan Q, Kausch, Henning H. Degradation on freezing dilute polystyrene solutions in p-xylene /J Polym. Sci., Part B: Polym. Phys 1994;32;1257-69. |
| [26] | Zubow KV, Zubow AV, Zubow VA. Study of structural changes in a LDPE film by flicker- noise spectroscopy. Plasticheskie Massy 2006;3;32-4, in Russian. |
| [27] | Zubow KV, Zubow AV, Zubow VA. Spectra of seed cristalls oscillation of NaCl in water solution. J. Appl. Spectr 2005;72; 766-72. |
| [28] | Zubova KV, Zubov AV, Zubov VA. Low frequency movement of cluster-12 in potato amylopectin during growth. Influence of white noises. Khimiya Rastitel'nogo Syr'ya 2009;2; 77-87 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML