-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2012; 2(1): 12-15
doi:10.5923/j.pc.20120201.03
Synthesis and Characterization of Surfactant Stabilized Trimetallic Au-Ag-Pd Nanoparticles for Heck Coupling Reaction
P. Venkatesan, J. Santhanalakshmi
Department of Physical Chemistry, University of Madras, Guindy Campus, A.C.Tech., Chennai, 600025, Tamil Nadu, India
Correspondence to: P. Venkatesan, Department of Physical Chemistry, University of Madras, Guindy Campus, A.C.Tech., Chennai, 600025, Tamil Nadu, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
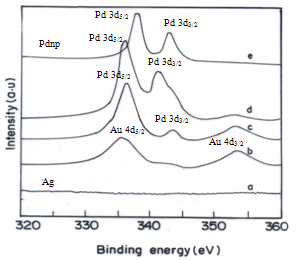
Colloidal dispersions of trimetallic nanoparticles (tnp) are synthesized by chemical method and compositionally four different tnp are prepared and particle sizes are characterized by UV-vis, HRTEM and XPS measurements. The XPS studies provide proof of the mode of binding that occurs in the nanoparticle surface. The catalytic activities of tnp are tested by Heck C-C coupling reaction. The product yields and recyclability of the recovered catalysts are studied. Tnp exhibited better catalysis than mono and bimetallic nanoparticles, which may be due to electronic effects of the Au-Ag core on to the Pd shell atoms.
Keywords: Trimetallic Nanoparticles, XPS Study, Core-Shell Nanoparticles, C-C Coupling Reaction, Gas Chromatography, Catalysts
Cite this paper: P. Venkatesan, J. Santhanalakshmi, Synthesis and Characterization of Surfactant Stabilized Trimetallic Au-Ag-Pd Nanoparticles for Heck Coupling Reaction, Physical Chemistry, Vol. 2 No. 1, 2012, pp. 12-15. doi: 10.5923/j.pc.20120201.03.
Article Outline
1. Introduction
- Colloidal dispersions of bi and multi metallic nanoparticles have garnered a lot of attention in recent years, particularly in the areas of optoelectronic applications and catalysis[1-5]. In the realm of optoelectronic applications, metal nanoparticles have interesting size and shape- dependent optical and electronic properties that can be suitably modulated by the addition of another metal[6]. For catalytic applications, the expectation is that they will have better catalytic properties than the component metals alone or even have new properties[7]. Chemical preparation of these colloidal multi metallic systems usually involves simultaneous and sequential reduction of the precursor ions and latter usually gives rise to core-shell structures. In both cases polymers, surfactants, ligands are added during the reduction process to prevent aggregation of the formed colloidal nanoparticles[8-10].Chen-Sheng Yeh et al. were among the first to use laser irradiation to synthesize trimetallic nanoparticles[11]. They used a Nd:YAG pulsed laser operated at a 10 Hz to synthesize tnp from aqueous solutions containing gold, silver and palladium. In our group, Santhanalakshmi et al. have reported that to prepare mono metallic Au, Ag, Pd, and bimetallic Au-Ag, Ag-Pd, and Au-Pd core-shell nanoparticles by chemical reduction of metal ions in aqueous solution [12,13], particle sizes and distribution are governed by the reduction rate, with faster rates leading to smaller and more uniform particles. Haverkamp et al. have synthesized tnp of gold, silver and copper with a narrow size distribution by irradiation of an aqueous solution containing both Au(III), Ag(I) and Cu(II) ions. They reported alloy like core-shell morphology for the resulting nanoparticles with a Cu shell over the Au, Ag core tnp with Au core, Ag and Cu shell have also been synthesized by Haverkamp et al. using successive irradiation of the precursors copper chloride, silver nitrate and gold chloride in plants materials[14]. Xin Zhag et al. have also reported the synthesis of tnp of Pt-Ru-Mo in the presence of microemulsions[15], Au-Pt-Rh in the presence of poly(N-vinyl-2-pyrrolidone)[16] and Zhang et al have also prepared Pt–Ru–Co trimetallic clusters by the reduction of an aqueous Pt–Ru–Co precursor solution with a parallel micro-emulsion system[17].In this paper, the synthesis of Au-Ag-Pd trimetallic nanoparticles in the chemical method by the simultaneous reduction of HAuCl4, AgNO3 and H2PdCl4 with reducing agent was studied using CTAB as the capping agent. The size, structure, optical properties and composition distribution of the resultant nanoparticles were characterized by UV-vis, TEM and XPS. The catalytic efficiency of the environmentally benign Pdnp and that of the four compositionally different tnp are monitored by Heck coupling reaction shown in Scheme 1. The products yield, reaction time and recyclability of the catalyst are the parameters derived from the reaction conducted separately using the Pdnp and four tnp catalysts.
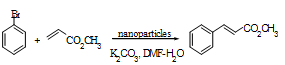 | Scheme 1. |
2. Experimental
2.1. Preparation of Au-Ag-Pd trimetallic nanoparticles
- Our laboratory recently developed a methodology for sequential reduction and preparing core-shell nanoparticles by wet chemical method of mixtures consisting three metal colloids, e.g. Au–Ag–Pd [18]. In view of our successful preparation of tnp, we took the next step and conducted the first trial of simultaneous reduction of three metals in chemical methods in Au–Ag–Pd colloidal mixtures.The colloidal dispersions of surfactant protected tnp were prepared by refluxing of the aqueous solution of HAuCl4, AgNO3 and PdCl2 in the presence of CTAB. The colloidal dispersion of 1:1:1 precursor metal salts are mixed at refluxed temperature in the designated ratio at one hour and the molar ratio of monomer unit of CTAB against total metal (R) were kept 30 in the present experiments. The mixed dispersions are kept stirring at least for a 24 hrs at room temperature to complete the self-organization reaction. The tnp with different compositions molar ratio (1:1:0.5, 1:0.5:1 and 0.5:1:1) of the precursor metal salts of gold, silver and palladium could be prepared without any precipitation above the similar method. The solutions of all the compositions are very stable over extended periods of time.
2.2. Heck coupling reaction - Catalytic study
- Methyl acrylate (1.05 mmol), bromobenzene (1.0 mmol), K2CO3 (3.0 mmol) and 15 mL of 3:1 v/v DMF-water solvent mixture are added into a three necked round bottomed flask with continuous stirring under N2 atmosphere. 0.5 mol % nanoparticle catalysts are added and temperature is maintained at 120℃. The progress of the reaction was monitored with gas chromatography. After completion of the reaction, 0.1 M HCl solution and 4-fold excess of CH2Cl2 are added so that, the nanoparticles catalyst and the products are correspondingly separated into the pH responsive aqueous and organic phases respectively. The catalyst was collected by ultracentrifugation and reused for the next cycle of coupling reaction.
3. Results and Discussion
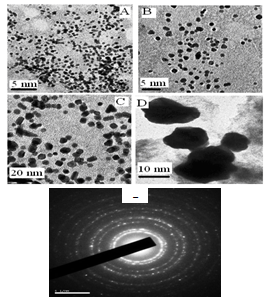
- The completion of formation reactions of mono metallic gold, bimetallic Au-Ag and trimetalli Au-Ag-Pd (1:1:1 mole ratio) nanoparticles were followed by UV-vis spectra as a function of time (Fig.1). The absorption spectra of the mixtures changed as the duration time was increased, the surface plasmon resonances peak (spr) bands moved closer to one another and merged into a single peak over the course of the time intervals. The mono metallic gold nanoparticles as conformed within 7 min and the bimetallic Au-Ag nanoparticles as conformed after 50 min, further increased the time duration resulted in a continuous decrease in the absorption band until, eventually, no absorption peak was apparent after 60 min exposure. As the duration of the time was increased upto 24 hrs, there is no distinct change occurred in the shape of the absorbance.Finally the color of the colloidal nanoparticles is dark brown as conformed formation of palladium coated trimetallic nanoparticles. The Pd nanoparticle shows a sharp absorbance peak cannot be observed over the entire range due to the brown colour of the solution. Successive reduction strategies are an effective method to prepare core-shell structure of bi and multi metallic nanoparticles. One of the metal salts is reduced first to form the core, and then a second metal is deposited on the surface of pre-formed monometallic nanoparticles to form the shell.
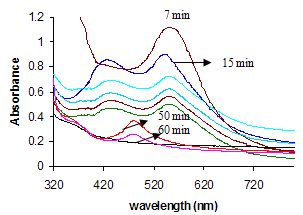 | Figure 1. UV-Vis absorption spectra of CTAB stabilized trimetallic Au-Ag-Pd colloidal nanoparticles at different time intervals in aqueous medium at 25℃ |
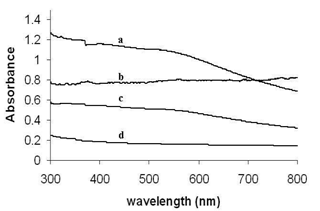 | Figure 2. UV-Vis absorption spectra of CTAB stabilized trimetallic nanoparticles of various concentrations, (a) 1:1:0.5, (b) 1:0.5:1, (c) 0.5:1:1, and (d) 1:1:1 respectively in aqueous medium at 25℃ |
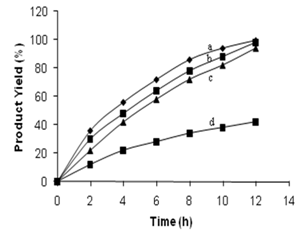 | Figure 5. Comparison of the catalytic activity of Au/Ag/Pd trimetallic nanoparticles (a = 1:1:1, b = 1:1:0.5), mono metallic Pd nanoparticles (c) and absence of nanoparticles (d) |
4. Conclusions
- In conclusion, we have synthesized a novel family of Au-Ag-Pd core-shell trimetallic nanoparticles from chemical method of metal-surfactant complexes has been reported. Surface plasmon peaks in UV-vis spectroscopy monitored the formation of mono, bi and trimetallic nanoparticles. The TEM photographs of tnp showed the sizes as 13 ± 0.5 nm. The particle size results from TEM agree well with each other. XPS spectra confirmed the metallic state of the colloidal nanoparticles. Their catalytic activities were tested in the Heck coupling reactions. This novel palladium containing tnp catalysis of Heck reaction produced better results than mono and bimetallic catalysts. Intact, presence of metal nanoparticles as catalysts replaces enviro-deadlier catalytic strong bases being used in Heck reaction. The high catalytic activity of trimetallic nanoparticles is probably due to the sequential electronic effect between elements in a particle. More detailed investigations of nanoparticle structure effects on the catalytic activity and their applicability in other synthetic transformations are currently under investigation.
ACKNOWLEDGMENTS
- The authors thank financial support to the Department from DST-FIST.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML