-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2012; 2(1): 6-11
doi: 10.5923/j.pc.20120201.02
Development and Validation of the Quantitative Analysis of Ceftazidime in Powder for Injection by Infrared Spectroscopy#
Andréia de Haro Moreno , Hérida Regina Nunes Salgado
Department of Drugs and Medicines, School of Pharmaceutical Sciences, University of São Paulo State, Araraquara, 14801-902, Brazil
Correspondence to: Hérida Regina Nunes Salgado , Department of Drugs and Medicines, School of Pharmaceutical Sciences, University of São Paulo State, Araraquara, 14801-902, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ceftazidime quantification by the infrared spectroscopy was developed and validated for pharmaceutical preparations in powder for injection. This method involved absorbance measurements of the band corresponding to aromatic ring centered by 1475-1600 cm-1. Selectivity, linearity, precision and accuracy were determined in order to validate the proposed method. It was also found that the excipient did not interfere with the assay. Calibration curve was obtained for ceftazidime at 0.5 to 7.0 mg, and mean recovery percentage was 98.98 ± 0.70. The proposed method was successfully applied to the assay of ceftazidime in powder for injection.
Keywords: Ceftazidime, Infrared, Spectroscopy, Quantitative, Determination
Cite this paper: Andréia de Haro Moreno , Hérida Regina Nunes Salgado , "Development and Validation of the Quantitative Analysis of Ceftazidime in Powder for Injection by Infrared Spectroscopy#", Physical Chemistry, Vol. 2 No. 1, 2012, pp. 6-11. doi: 10.5923/j.pc.20120201.02.
Article Outline
1. Introduction
- Ceftazidime (Fig. 1) is a third-generation cephalosporin that is widely used for the treatment of serious infections caused by Gram-negative bacteria, including Pseudomonas aeruginosa. They include biliary-tract infections, bone and joint infections, cystic fibrosis (respiratory-tract infections), endophthalmitis, infections in immunocompromised patients (neutropenic patients), meningitis, peritonitis, pneumonia, septicaemia, skin infections (including burns, ulceration) and urinary-tract infections[1-13].
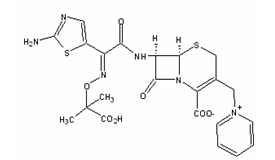 | Figure 1. Chemical structure of ceftazidime – C22H22N6O7S2 (mw 546.58) |
2. Material and Methods
2.1. Chemicals
- Ceftazidime reference substance (assigned purity 99.98%) and ceftazidime powder for injection were kindly supplied by Ariston Química e Farmacêutica Ltda. (São Paulo, Brazil). Ceftazidime powder for injection (Ceftazidon) was claimed to contain 1000 mg (as anhydrous base) of the drug and 118 mg of anhydrous sodium carbonate as excipient (solubilizer).Potassium bromide (Merck, Darmstadt, Germany) used to the preparation of translucent pellets was of analytical grade and was previously dried at 120ºC for 2 h.
2.2. Instrumentation and Analytical Conditions
2.2.1. Equipment
- A conventional SHIMADZU IR Spectrometer Model FTIR 8300 (Tokyo, JP) with spectral digitalization was used for obtaining data and respective absorption regions (wavelength region of 500-4000 cm-1 at 2 cm-1 intervals).
2.2.2. Obtaining of Analytical Curve
- Translucent pellets were prepared by dilution of ceftazidime reference substance in potassium bromide to obtain 250 mg of total weight. Amounts of 0.5, 1.0, 2.0, 5.0 and 7.0 mg of ceftazidime reference substance (previously diluted with potassium bromide 1:10, w/w) were prepared using sufficient amount of potassium bromide to obtain 250 mg. Powders were mixed and ground until the obtaining an homogeneous powder; so, this powder mixture was crushed in a mechanical die press to form translucent pellets through which the beam of the spectrometer can pass.
2.2.3. Sample Preparation
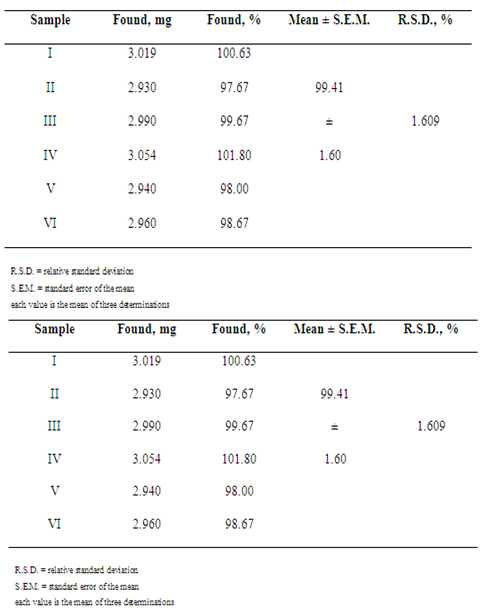
- Twenty flasks containing ceftazidime powder for injection were weighed and the average weight was determined. An amount of powder equivalent to 300 mg of ceftazidime was mixed and ground with potassium bromide for the obtaining the homogeneous powder (1:10, w/w dilution). Dilutions with potassium bromide were made to give final concentrations of 3.0 mg in each translucent pellet. This dilution procedure was performed in triplicate. Lectures were made at wavelength region of 500-4000 cm-1 and the absorbance measurements were monitored at 1475-1600 cm-1 region, that corresponding to the aromatic ring absorption region of the molecule.
2.2.4. Sample Preparation
- Sample concentrations (mg) were calculated by following: CS =[AS x CRS] / ARS, where CS is the sample concentration (mg), CRS is the reference substance concentration (mg), AS is the sample absorbance measurement and ARS is the reference substance absorbance measurement.
2.2.5. Method Validation
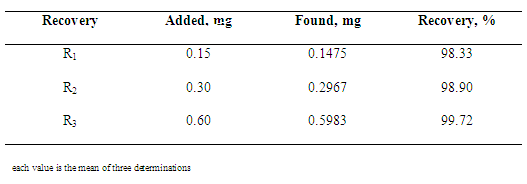
- The developed method was validated by the following parameters: linearity, precision and accuracy as per ICH Guidelines[51] and AOAC[52].a) Linearity: To assays linearity of the method, doses of reference substance were evaluated on 3 different days. Regression lines were calculated by the least-squares method. Statistical evaluation was made by ANOVA. For the infrared spectrometric method, linearity was verified by analysis of 5 points at concentration range 0.5-7.0 mg. b) Precision: Repeatability (intraday) and intermediate precision (inter-day) were evaluated by the assay of 6 independent samples in a day, under the same experimental condition (standard and sample preparation as described above). Results obtained on 3 different days were compared.c) Accuracy: This parameter was determined by the recovery study, comparing theoretical and measured concentrations of ceftazidime reference substance added at the beginning of the process. Translucent pellets were prepared at concentration 3.0 mg in each pellet. Amounts of 30.0 mg of ceftazidime sample dilution (1:10, w/w) in potassium bromide and amounts of 15.0, 30.0 and 60.0 mg of ceftazidime reference substance, previously diluted in potassium bromide (1:100, w/w), corresponding to 150, 300 and 600 µg of ceftazidime, respectively, were weighed, mixed and ground with sufficient amount of potassium bromide for the obtaining the homogeneous powder (250 mg each pellet), giving final concentrations of 3.15, 3.30 and 3.60 mg in each pellet, respectively, which correspond to 105, 110 and 120% (R1, R2 and R3) of nominal analytical concentration. Each level was made in triplicate.Recovery percentage of ceftazidime added was calculated using the equation proposed by AOAC[52].
3. Results and Discussion
- Quality control is very important to guarantee the safety and the effectiveness of pharmaceuticals. In order to control the production line as best as possible and to increase the productivity a lot of samples were drawn and analysed in certain intervals. But still the test procedure solely rely on random testing, because it was up to now the only way to assure the quality of the millions of products produced in a day. Clusters of products, faulty in constituents, concentration or humidity, caused by momentary production problems could not always be detected[53].Near-infrared spectroscopy is a widely recognized technique for identification and verification of compounds. It is non-contact, non-destructive and no sample preparation is required[54]. This technique has been used to identify several compounds, such as pharmaceuticals, cosmetics and foods, but requires expensive equipments and mathematical pre-treatments[54, 55, 56]. So, the aim of this study was developed an infrared spectrometric method for the determination of ceftazidime in powder for injection.Infrared spectra obtained presented characteristic absorption bands of cephalosporin compounds, such as 3660-3250 cm-1 (N-H group axial deformation), 1750-1725 cm-1 (carboxylic acid function C=O stretching), 1475-1600 cm-1 (aromatic ring C=C axial deformation), 1350-1300 cm-1 (C-N axial deformation) and 1680-1630 cm-1 (amide group C=O axial deformation), according to Figure 2.Spectra showed characteristic absorption bands and absorbance measurements of them; so, it was possible to verify the linear relationship between ceftazidime concentrations and absorbance measurements when different amounts of the drug were used to prepare the translucent pellets. Spectra were analysed and compared to that obtained of ceftazidime reference substance for identify the sample. Absorption bands of aromatic ring were chosen for absorbance monitoring because those absorption bands did not occur in excipient present in pharmaceutical preparations (anhydrous sodium carbonate or arginine).Infrared spectroscopic method presented many advantages when compared to both UV/Vis spectrophotometric methods: there wasn’t necessary to realize extraction stages, which reduced time analysis and costs with filter equipments and membranes. Besides, drugs presenting solubility problems with more appropriate solvent could be prepared in powder form (generally, potassium bromide) for obtaining the pellets. Time procedure was also smaller than that solutions preparation.Excipients present in pharmaceutical preparation (powder for injection) did not interfere with the results obtained because those do not present specific absorption bands used to identify and quantify the analysed drug.Despite being only moderately selective, infrared spectroscopic method was a very robust, easy and inexpensive method when compared to other instrumental methods, offering good precision in quantitative analysis.Calibration curve of ceftazidime was obtained by plotting absorbance measurements against drug concentration. The curve was linear at range 0.5-7.0 mg, with a regression coefficient of 0.9998 and a linear regression equation of y = 0.1717x + 0.2129 (Figure 3).
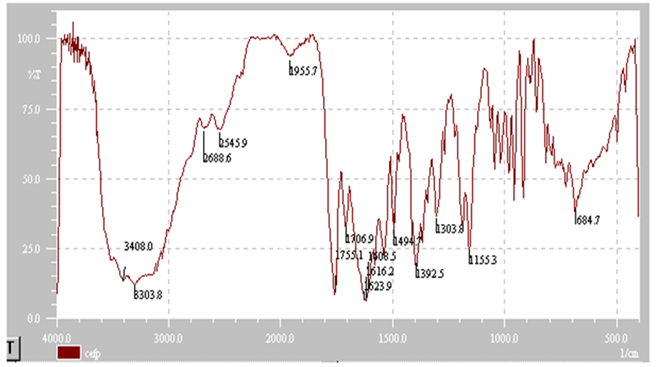 | Figure 2. Infrared spectrum for ceftazidime reference substance |
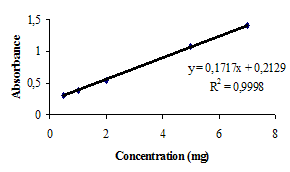 | Figure 3. Calibration curve for ceftazidime by infrared spectroscopic proposed method |
|
|
3. Conclusions
- Ceftazidime quantification in powder for injection by the infrared spectroscopic method demonstrated good linearity, precision and accuracy at concentrations ranging from 0.5 to 7.0 mg. The present investigation showed that infrared analysis could be also employed for quantitative determination of ceftazidime in pharmaceutical preparations, with possible application for quantification of other drugs. Therefore, it was an acceptable alternative method for the routine quality control of ceftazidime in raw material and pharmaceuticals. The proposed method used simple reagents, with minimum sample preparation procedures, encouraging its application in routine analysis.
ACKNOWLEDGEMENTS
- Authors thank Ariston Química e Farmacêutica Ltda. (São Paulo, Brasil) for providing ceftazidime reference substance and ceftazidime powder for injection. This work was supported by PACD-FCFAr-UNESP-Brazil, FUNDUNESPBrazil, FAPESP-Brazil and CNPq-Brazil.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML