-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Physical Chemistry
p-ISSN: 2167-7042 e-ISSN: 2167-7069
2011; 1(1): 14-16
doi: 10.5923/j.pc.20110101.03
Influence of Permanent Magnet on the Association Constants of FeCl3+10% PVA (Polyvinylalcohol) in 50% Ethanol-Water Solutions Conductometrically at 298.15K: Using New Equation for 1:3 Asymmetric Electrolytes
Nagah A. El-Shishtawi 1, Maany A. Hamada 2, Esam A. Gomaa 2
1Department of Physics
2Department of Chemistry, Faculty of Science, Mansoura University, 35516, Mansoura, Egypt
Correspondence to: Esam A. Gomaa , Department of Chemistry, Faculty of Science, Mansoura University, 35516, Mansoura, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The external poles of a permanent magnet of power 1.26 kG were used to study their effect on the conductance values of FeCl3 plus 10% by weight , PVA (polyvinylalcohol) in 50 % by weight mixed ethanol-water solutions. A new equation for 1:3 asymmetric association constant was derived and used for calculating the association constants (KA) for FeCl3 solutions in 50 % ethanol (EtOH)-H2O mixture in presence of PVA and also in absence and presence of an external magnetic field. The new equation was derived from the Fuoss-Shedlovsky equation and Ostwald dilution law and the evaluated values are discussed.
Keywords: Magnetic Effect, Conductance, FeCl3 , PVA Solutions , 50% Ethanol, Water , Association Constants
Cite this paper: Nagah A. El-Shishtawi , Maany A. Hamada , Esam A. Gomaa , "Influence of Permanent Magnet on the Association Constants of FeCl3+10% PVA (Polyvinylalcohol) in 50% Ethanol-Water Solutions Conductometrically at 298.15K: Using New Equation for 1:3 Asymmetric Electrolytes", Physical Chemistry, Vol. 1 No. 1, 2011, pp. 14-16. doi: 10.5923/j.pc.20110101.03.
1. Introduction
- Conducting polymers have received much attention due to their electroactive properties at electrode surface when they are doped by electrochemical oxidation-reduction reactions by chemical methods using electron donors or acceptors(1,2). These properties allow the polymers to be used as modifiers of electrode surface to facilitate organic electrochemical reaction at the electrodes and to improve battery electrodes(3).Adding cations as doped elements improve the conducting properties of polymers and also play an important role in forming thin films(3).Conductivity is a good method for explaining the ion-interactions and the association of ions in different solutions(4). In this work we apply new equation for the association constants for 1:3 asymmetric electrolytes by the use of conductivity data. This new equation is derived from Fuoss-Shedlovsky equation and Ostwald dilution law(5-8).Studying the effect of external magnet on the association constants of FeCl3+10% PVA+50% ethanol-water solutions is very important because it is not studied before.
2. Experimental
- Ferric chloride was provided from Merck and ethanol BDH was used without purification. Polyvinylalcohol, M.W, 17,000 water soluble polymer, from Arondale laborations, was used. 5 ml of mixed 50% ethanol-H2O were put in test tubes then different salt concentrations of FeCl3+10% PVA by weight were added and dissolved. The prepared solutions were left for two days in water thermostate of the type (Polyscience 8105, USA) at 298.15 K to reach the necessary equilibrium. Necessary volumes were withdrawn and measured using density techniques, conductivity and capacitance.The density measurements were done by taking 1 ml of the prepared solutions and put in specific gravity bottle (1 ml capacity) and weighing them using Mettler-Toledo USA, four digital weighing balance. The density have been used to calculate the solvated radii(9,10).Conductances and capacitances were measured experimentally by the use of multimetter of the type [Macom (MX620)] with sensitivity of 1%. Dipping type cell with two carbon electrodes apart with 1 cm distance and with cell constant equal 0.96, was used. At least three readings were done. The conductance values of all solutions were corrected by substracting their values from that of pure solvents. The required temperature was adjusted at 298.15 K with a precision of ±1 K.Two poles of permanent magnet was used with power 1.26 K Gauss (kG) measured by Gauss metter Model GM-54. The measured solutions were put between the two poles of the magnet and their conductivity were measured.
3. Results and Discussion
- From the densities of FeCl3+10% PVA in mixed 50% ethanol-water solutions at 298.15 K, the molar volumes were calculated by dividing the molecular weight of FeCl3 by the densities of 50% mixed ethanol-H2O solutions and the evaluated volume values are represented in Table 1, in presence of 10% PVA. From the molar volumes, the solvated radii rs in Å units for FeCl3+50% (EtOH-H2O) in presence and absence of PVA were calculated by using equation 1.
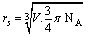 | (1) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
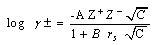 | (2) |
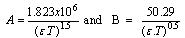 | (3) |
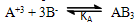 | (3) |
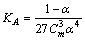 | (4) |
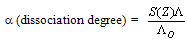 | (5) |
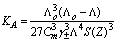 | (6) |
 | (7) |
 = 0, the limiting molar conductivity Λo was obtained.The same relation was obtained from the measured specific conductivities in presence of two poles of permenant magnet of power 1.26 K. Gauss.From Λ, Λo values, the association constants (KA) for FeCl3+50% (EtOH-H2O)+10% PVA solvents in presence and absence of magnetic field were calculated by applying equation (7) and their data are presented in Table 2.The dissociation degrees (α) were also evaluated for FeCl3+50% (EtOH-H2O)+10% PVA in absence and presence fo magnetic field (see Table 2) and for FeCl3+50% EtOH-H2O+10% PVA in absence and presence of magnet (see Table 2).It was observed that KA) values decrease with increase FeCl3 concentrations due to the decrease in the dissociation degree. PVA favour very high FeCl3 associations, i.e., increase the KA values. This prove that the association of electrolyte ions took place easier through polymer media. Also the magnet increase also the association constants of FeCl3+50% EtOH-H2O+10% PVA which indicate that the magnet attract the cations and therefore facilitate the association through its surface.
= 0, the limiting molar conductivity Λo was obtained.The same relation was obtained from the measured specific conductivities in presence of two poles of permenant magnet of power 1.26 K. Gauss.From Λ, Λo values, the association constants (KA) for FeCl3+50% (EtOH-H2O)+10% PVA solvents in presence and absence of magnetic field were calculated by applying equation (7) and their data are presented in Table 2.The dissociation degrees (α) were also evaluated for FeCl3+50% (EtOH-H2O)+10% PVA in absence and presence fo magnetic field (see Table 2) and for FeCl3+50% EtOH-H2O+10% PVA in absence and presence of magnet (see Table 2).It was observed that KA) values decrease with increase FeCl3 concentrations due to the decrease in the dissociation degree. PVA favour very high FeCl3 associations, i.e., increase the KA values. This prove that the association of electrolyte ions took place easier through polymer media. Also the magnet increase also the association constants of FeCl3+50% EtOH-H2O+10% PVA which indicate that the magnet attract the cations and therefore facilitate the association through its surface.
References
| [1] | E.G. Lyon (Ed.), Electroactive Polymer Electrochemistry, Part 1, Plenum Press, New York, 1994 |
| [2] | H.S. Nalwa (Ed.), handbook of Organic Conductive Molecules and Polymers, Vols. 1-4, Wiley, Chichester, England, 1997 |
| [3] | Dong-Hun Han, Hyo Joong Lee and Su-Moon Park, Electrochimica Acta, 50, 3085, 2005 |
| [4] | U.N. Dash, J.R. Mahapata and B. Lal, Journal of Molecular Liquids, 124, 13, 2006 |
| [5] | T. Shedlovsky, R.L. Kay, J. Phys. Chem., 60, 51, 1956 |
| [6] | R.M. Fuoss, J. Phys. Chem., 79, 525, 1975 |
| [7] | R.M. Fuoss, J. Phys. Chem., 81,1829, 1977 |
| [8] | A.K. Covingtion and T. Dichinson, “Physical Chemistry of Organic Solvent Systems”, Plenum Press, London, 1973 |
| [9] | E.A. Gomaa, Proc. Kon. Neder. Ak. Van. Weten, 91B, 363, 1988 |
| [10] | E.A. Gomaa,Ind. J. of Tech., 26, 461, 1988 |
| [11] | Esam A.Gomaa, Rev. Roum. De Chim., 39, 1253, 1991 |
| [12] | J.I. Kim, A. Cecal, H.J. Born and E.A. Gomaa, Z. Phys. Chem. Neue Folge, 110, 209, 1978 |
| [13] | E.A. Gomaa, M.A. Hafez and M.N.H. Moussa, Bull. Soc. Chim. Fr., 3, 361, 1986 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML