-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2019; 8(2): 25-29
doi:10.5923/j.otolaryn.20190802.03

Rhino-Orbito-Cerebral Mucormycosis: Our Experience
Karthik Shamanna 1, Afshan Fathima 2, Sowjanya S. 3
1Professor, Department of Otorhinolaryngology, Bangalore Medical College & Research Institute, Bangalore, India
2Senior Resident, Department of Otorhinolaryngology, Bangalore Medical College & Research Institute, Bangalore, India
3Junior Resident, Department of Otorhinolaryngology, Bangalore Medical College & Research Institute, Bangalore, India
Correspondence to: Afshan Fathima , Senior Resident, Department of Otorhinolaryngology, Bangalore Medical College & Research Institute, Bangalore, India.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

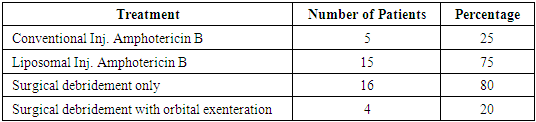
Introduction: Mucormycosis is an acute, invasive and often fatal infection caused by fungi belonging to the class zygomycetes. The most common organism causing fungal infection of the nose and paranasal sinuses is rhizopus. It is more commonly encountered in patients with immunocompromised status such as diabetic ketoacidosis, immunosuppression, prolonged usage of corticosteroids etc. Aims & Objectives: 1) To study the clinical profile of patients diagnosed with rhino-orbito-cerebral mucormycosis. 2) To determine the importance of early detection and management of patients with rhino-orbito-cerebral mucormycosis. Materials & Methods: The present study was conducted at the department of ENT, Bangalore Medical College and Research Institute, Bangalore. 20 patients clinically diagnosed as rhino-orbital/rhino-cerebral mucormycosis were included in the present study between April 2018 to September 2019. The diagnosis of rhino-orbito-cerebral mucormycosis was based on clinical, radiological, microbiological and endoscopic findings. Patients were treated with systemic antifungal therapy along with endoscopic debridement of necrotic material within the nose and paranasal sinuses. Patients were also treated for their underlying immunocompromised condition if any and followed up for 6 months after resolution of symptoms. Results: Males (65%) were affected more frequently than females in our case series. The most common age group affected was between 41-50 years (40%). 15(75%) of our 20 patients had diabetes mellitus as the underlying predisposing factor. 15(75%) of our 20 patients were treated with liposomal Inj Amphotericin B along with surgical debridement which had good results in terms of disease clearance and lesser complications. 5 (25%) patients treated with conventional Inj Amphotericin B had more complications such as hypokalemia, acute kidney disease, hypomagnesemia etc. 3(15%) patients died in the follow up period. Conclusion: Early detection and prompt treatment are vital in the management of rhino-orbito-cerebral mucormycosis as the disease spreads rapidly and is often fatal. Treatment with liposomal Inj Amphotericin B along with surgical debridement of necrotic tissue should be carried out in all cases for adequate clearance of the disease. When there is spread of disease to orbit, orbital exenteration should be done for complete clearance of disease and to prevent intracranial spread of infection.
Keywords: Rhino-orbito-cerebral, Mucormycosis, Amphotericin B, Debridement
Cite this paper: Karthik Shamanna , Afshan Fathima , Sowjanya S. , Rhino-Orbito-Cerebral Mucormycosis: Our Experience, Research in Otolaryngology, Vol. 8 No. 2, 2019, pp. 25-29. doi: 10.5923/j.otolaryn.20190802.03.
1. Introduction
- Mucormycosis is defined as an infection caused by fungi belonging to the order Mucorales and class Zygomycetes [1]. It was first described by Paultauf A in 1885. The most important species causing the infection is rhizopus arrhizus. Amongst the fungal infections, mucormycosis is the most invasive form leading to higher morbidity and mortality [2]. Mucorales enter the body via inhalation, percutaneous inoculation or ingestion of spores.Mucormycosis can manifest in four variants: rhinocerebral, pulmonary, gastrointestinal and disseminated [3]. It most commonly manifests in the paranasal sinuses (39%) followed by lungs (24%), skin (19%), brain (9%), gastrointestinal tract (7%) and disseminated form (6%). The risk factors implicated in the development of mucormycosis are diabetic ketoacidosis, iatrogenic immunosuppression, neutropenia, use of corticosteroids or desferoxamine and disruption of mucocutaneous barriers by catheters and other devices contaminated by these fungi [4-7].Increased glucose content within the tissues or subsequent acidosis as encountered in diabetic patients serves favorable to the growth of rhizopus. Factors such as high pH, high temperature and high glucose content favor the growth of the organism [8,9]. Presence of altered leucocyte function and impaired accumulation of granulocytes within the tissues, as seen in ketoacidosis can further lower the patient’s natural immunity which in turn facilitates the growth of the opportunistic fungi [10].The rhino-cerebral variant comprises 33-50% of all cases of mucormycosis [11,12]. Clinical manifestations may start with necrosis of the palate or within the paranasal sinuses, which may progress towards the orbit before reaching intra-cranial structures. The most frequent symptoms include headache, nasal block, unilateral facial swelling, facial pain or visual disturbances. Thrombosis of the cavernous sinus and cranial nerve invasion may be a result of unresolved rhino-orbital mucormycosis. Presence of an eschar on the palate or nasal mucosa is mostly suggestive of mucormycosis although it may be absent in about 50% of the cases [13]. Factors responsible for poor prognosis include a delay in treatment, intra-cranial involvement, bilateral sinonasal involvement, invasion of the palate and the presence of multiple underlying immunocompromised conditions [14].Aims & Objectives1) To study the clinical profile of patients diagnosed as rhino-orbito-cerebral mucormycosis.2) To determine the importance of early detection and management of patients with rhino-orbito-cerebral mucormycosis.
2. Materials & Methods
- The present study titled ‘Rhino-Orbito-Cerebral Mucormycosis: Our Experience.’ was conducted at the department of ENT, Bangalore Medical College & Research Institute, Bangalore, between April 2018 and September 2019.Study design: Retrospective case series.Sample size: 20 patients.Statistical Analysis: data was collected and tabulated in an excel sheet. Results presented as percentages and proportions.Methodology: 20 patients clinically diagnosed with rhino-orbito-cerebral mucormycosis were included in the present study. Detailed clinical history regarding onset and progression of symptoms, clinical examination and diagnostic nasal endoscopy were performed in all patients. The eschar within the nose and paranasal sinuses was detected for fungal elements using a KOH mount. This was followed by radiological evaluation with computed tomography (CT) of nose and paranasal sinuses which was done in all patients and MRI brain with orbit was done when intracranial or intraorbital infection was suspected. Ophthalmologist and neurologist opinion was taken where necessary.After an initial test dose, patients were started on systemic Inj Amphotericin-B. Conventional Amphotericin-B at 1mg/kg/day was started in patients who were not affordable for the liposomal variant. Most of our patients were treated with liposomal Inj Amphotericin-B at 3-5mg/kg/day as it was associated with lesser complications and showed better response. Patients were monitored for nephrotoxicity, electrolyte imbalance and blood glucose levels. Any deficits noted were corrected accordingly.Patients underwent surgical debridement of the necrotic material within the nose and paranasal sinuses. Patients who had an orbital involvement underwent exenteration to limit the infection within the orbit and prevent its spread intracranially. Complete clearance of the eschar on nasal endoscopy and a KOH mount negative for fungal elements was taken as an indication to stop Inj Amphotericin-B. Patients were followed up as an outpatient for 6 months.
3. Results
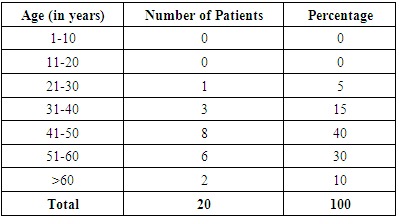
- 20 patients clinically diagnosed and managed as rhino-orbito-cerebral mucormycosis at our ENT department were included in the present study. Of the 20 patients, 13(65%) were males and 7(35%) were females (Graph 1). 41-50 years was the most common age group affected having 8(40%) out of 20 patients followed by 51-60 years age group with 6(30%) patients (Table 1).
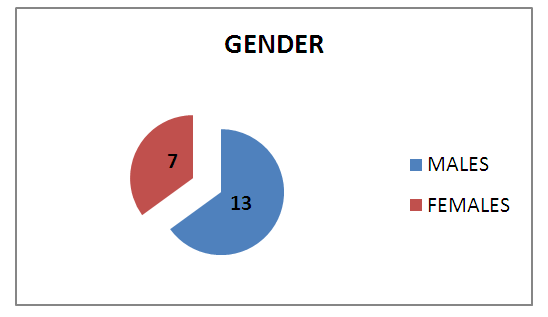 | Graph 1. Gender Distribution |
|
|
 | Picture 1. Ptosis & Ophthalmoplegia of left eye with broadening of nasal bridge- RHINO-ORBITAL MUCORMYCOSIS |
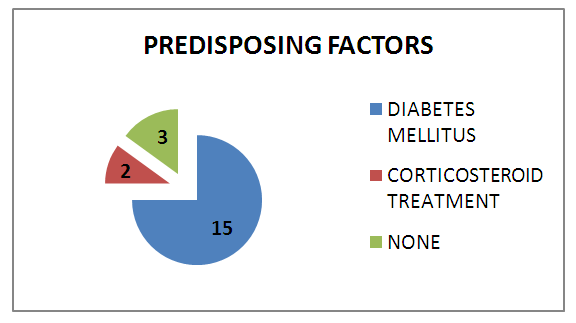 | Graph 2. Predisposing Factors |
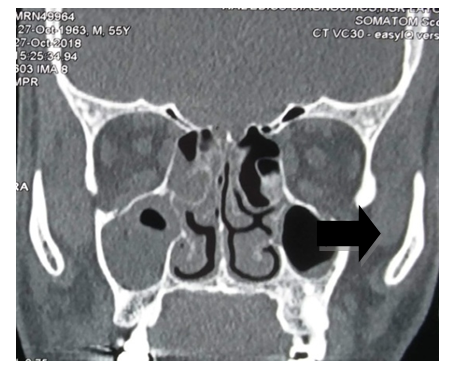 | Picture 2. CT NOSE & PNS:-ill defined heterogenous and minimally enhancing soft tissue density noted within the right maxillary sinus & nasal cavity-MUCORMYCOSIS |
|
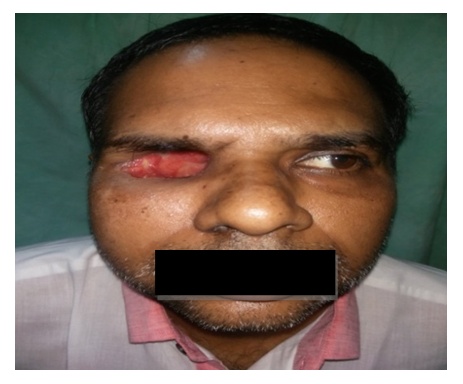 | Picture 3. Patient in follow-up period after left orbital exenteration was done for rhino-orbito- cerebral mucormycosis |
4. Discussion
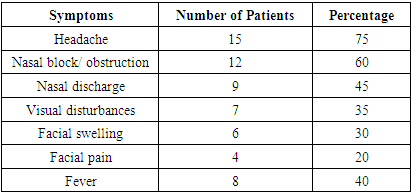
- Mucormycosis is one of the most rapidly progressive and invasive fungal infection in humans having a mortality rate of 70 to 100% [15]. In our study, male patients (65%) were more compared to female patients (35%). Most of the patients in our study were in the 4th decade (40%) and 5th decade (30%). Our results were similar to a study conducted by Roden et. al. [16], in which median age of patients was 40years.Initial symptoms mimicked sinusitis in 85% of our patients. Headache was the most common symptom in our study population (75%). The other symptoms observed were nasal block, facial swelling, nasal discharge, fever, visual disturbance etc. In a similar study done by Pafrey et. al. [17] and Ferry et. al. [18] showed a similar clinical profile in their patients. 7(45%) patients in our study had fever, similar to the study conducted by Talmi et. al. [19] in which fever was present in less than half of their cases.Several studies have shown that about 70% of sinonasal cases are found in diabetic patients with ketoacidosis [20]. In a study on 126 patients with rhinocerebral mucormycosis by Strasser et. al. [21] 70% patients were diabetic. Our study showed a slightly higher percentage of patients (85%) with diabetes. The high association of rhino-orbito-cerebral mucormycosis in diabetes mellitus and ketoacidosis could be attributed to the innate active ketone reductase system present in rhizopus that helps it thrive in high glucose and acidotic conditions. Also there is increased availability of serum iron in these conditions which favor the growth of the organism.The initial imaging study in mucormycosis is CT scan of nose and paranasal sinuses. Features such as heterogenous opacification and osteolytic changes go in favor of mucormycosis. This was the most common finding noted in our study population in 70% of our patients. A study conducted by Singh et. al. [22] showed unilateral sinus mucosal thickening as the most consistent finding. In advanced stages osteolytic changes involving sinus walls and heterogenous opacification with air pockets were observed.Nasal endoscopy plays a vital role to arrive at a provisional diagnosis in mucormycosis. Endoscopic examinations showing discoloration, ulceration of nasal mucosa or turbinates with eschar formation is highly suggestive of mucormycosis. In our study, we observed eschar formation within the nasal cavity and middle meatal region in 18 (90%) of the cases. Also erosion of hard palate was seen in 6 (30%) cases. Gillespie et. al. [23] in their study observed that 80% of patients developed necrotic lesion on either the nasal or oral mucosa.Patients with a clinical profile similar to mucormycosis should be started on treatment as early as possible. Studies conducted by Chamilos et. al. [24] and Spellberg B et. al. [25] showed that initiation of antifungal therapy within 5 days of diagnosis/ onset of symptoms had better prognosis when compared to late initiation of treatment i.e., 83% vs 49% survival. In our study, 14(70%) out of 20 patients presented early and were started on medical treatment. These patients showed better response to treatment and an earlier recovery.Endoscopic sino-nasal debridement forms a crucial part of treatment and was carried out in 18 (90%) of our patients. Debridement of the involved structures was done until clear bleeding margins were seen. The hallmark of mucormycosis is vascular invasion which leads to thrombosis of blood vessels and compromise of blood flow to surrounding tissues leading to avascular necrosis. This necrotic tissue harbors the fungi and facilitates further growth of the organism. This along with relatively poor vascularity of surrounding tissue impedes the reach of parenteral antifungal agents resulting in poor outcome. Hence surgical debridement is mandatory to remove all necrotic tissue till fresh bleeding is noted. This decreases the overall fungal load, increases the availability of antifungal agents to the viable tissue and results in better disease clearance. Patients require orbital exenteration in advanced cases with involvement of the orbit in order to limit the disease and prevent the spread of infection to the brain.
5. Conclusions
- Rhino-orbito-cerebral mucormycosis is a rapidly progressive disease with a fulminant course and fatal outcome unless diagnosed early and treated appropriately. Treatment with liposomal Inj Amphotericin B along with surgical debridement of necrotic tissue from nose and paranasal sinuses should be carried out in all cases for complete clearance of disease and to prevent the spread of disease into the orbit or intracranium. A multi-disciplinary approach consisting of dental specialists, otorhinolaryngologists, ophthalmologists and neurologist is critical in the successful management of a patient with rhino-orbito-cerebral mucormycosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML