-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2018; 7(3): 43-54
doi:10.5923/j.otolaryn.20180703.01

Assessment of Auditory Function in Obese Children
Asmaa Abd El Wakeel Elsehmawy1, Amal Mahmoud Hassan Ewida2, Amal Gaber Mohammed1, Nora Mohammed Ahmed Seliem3, Reham Yousri Elamir4, Alshimaa Mohamed Abdelmoaty4
1Department of Pediatrics, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
2Audio Vestibular Unit, ENT Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
3Biochemistry Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
4Department of Public Health, Faculty of Medicine, Cairo University, Egypt
Correspondence to: Asmaa Abd El Wakeel Elsehmawy, Department of Pediatrics, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Obesity is a chronic condition, associated with multiple comorbidities. Great attention has been paid to the obesity comorbidities such as cardiovascular disease and insulin resistance up to diabetes mellitus (T2DM), there’s evidence suggests that obesity affects hearing. Aim of the study: The aim of the current study was to evaluate the relationship between obesity and hearing in a group of obese children. Methods: The present study was a controlled study that was carried out on 80 children of both genders and divided into study group, 40 healthy obese children, they were subdivided after the study has been completed into; group I, 26 healthy obese children with normal middle ear function. Group II 14 obese children with middle ear dysfunction. Control group, included forty non-overweight children. Results: Our study revealedthat there were significant increase of the pure tone threshold in obese children with middle ear affection in comparison to obese with normal middle ear and the control group. Positive correlation between pure tone with the anthropometric measures, lipid profile and insulin resistance in obese children with diseased middle ear. Cochlear affection in obese children with normal middle ear evidenced by a negative correlation between transient otoacoustic emission with the anthropometry, liver enzymes and insulin resistance. Conclusion: Obesity considered as a risk for conductive hearing loss and cochlear affection.
Keywords: Cochlear affection, Obesity, Otitis media, Pure tone
Cite this paper: Asmaa Abd El Wakeel Elsehmawy, Amal Mahmoud Hassan Ewida, Amal Gaber Mohammed, Nora Mohammed Ahmed Seliem, Reham Yousri Elamir, Alshimaa Mohamed Abdelmoaty, Assessment of Auditory Function in Obese Children, Research in Otolaryngology, Vol. 7 No. 3, 2018, pp. 43-54. doi: 10.5923/j.otolaryn.20180703.01.
Article Outline
1. Introduction
- Obesity is a chronic condition, associated with multiple comorbidities that translate into reduced quality of life and decreased longevity. According to the World Health Organization, obesity is responsible for the death of 2.8 million people each year, worldwide [1]. Great attention has been paid to the obesity comorbidities such as cardiovascular disease and insulin resistance up to diabetes mellitus (T2DM), there’s evidence suggests that obesity affects hearing. High vascular and sensitive organs such as the auditory system can be affected by obesity that has significant effects on vascular function [2].Insulin resistance (IR) is the primary metabolic disorder associated with obesity and is defined as a diminished ability of insulin to stimulate glucose uptake by skeletal muscle and adipose tissue, in addition to reducing insulin's ability to suppress hepatic glucose production and output [3]. Among these, the homeostasis model assessment of insulin resistance index (HOMA-IR) is one of the most commonly used [4, 5] and insulin resistance is defined as values of a HOMA index greater than or equal 3.4 [6].Hearing impairment is usually a 'hidden disability' as its impact on an individual’s quality of life is rarely acknowledged, can be highly debilitating for an individual as impaired communication can result in a reduction in social inclusion, which may exacerbate obesity [7].Otitis media with effusion (OME) shows a higher morbidity profile in children, being a frequent cause of hearing loss. Childhood obesity has been associated with increased incidences of chronic infection, pulmonary immaturity and asthma. An association between child obesity and the occurrence of OME have been reported [8].OME are primarily caused by Eustachian tube (ET) dysfunction and bacterial infection, the underlying mechanisms of chronic and recurrent OME are not completely understood. Allergy is suggested to be a factor, as explained by the shock organ hypothesis. Various cytokines and inflammatory mediators secreted by mast cells induced by allergic reactions in the nasal and nasopharyngeal mucosae in turn can cause ET occlusion, resulting in OME [8]. In some studies, greater changes in middle ear pressure on nasal provocation can be demonstrated significantly in chronic secretory OM patients with allergic rhinitis compared with healthy individuals. Besides allergy, other factors can lead to chronicity of OME, including bacterial infection. Bacteria have low metabolic rates and produce biofilms and oxidative stress induced by ET dysfunction may be a source of persistent stimulation of infection. Such stimulation, as well as hydrops ex-vacuo, may induce chronic OME [9].Mediators of inflammatory responses, including proteins; peptides; glycopeptides; arachidonic acid metabolites, such as leukotrienes and prostaglandins; cytokines; oxidative nitrogen; and free radicals, are secreted by epithelium, endothelium, mast cells, infiltrating inflammatory cells and lymphocytes. These mediators can induce changes in vascular permeability and chemotaxis and can stimulate middle ear mucosa to secrete mycopolysacharides and other inflammatory mediators, thus playing important roles in the development of OME [10].Pro-inflammatory state and oxidative stress caused by excess macronutrients in the adipose tissues are predisposed by the release of inflammatory mediators such as tumor necrosis factor α and interleukin 6 and reduced production of adiponectin also the increased level of interleukin 6 stimulates the liver to synthesize and secrete C-reactive protein [10].The inner ear is a highly complex organ with similarly complex metabolic mechanisms. Changes in glucose and insulin blood concentration may cause hearing loss and vestibular disorders [11].Hypoxia and ischemic damage, oxidative stress and formation of reactive oxygen species, and resultant death of cochlear and spiral ganglion cells that leads to hearing loss may play as underlying mechanism that explain the relation between high BMI and hearing function. Stiffening and constriction of the internal auditory artery and reduction in cochlear blood flow may be caused by obesity related atherosclerosis [12].Reduced blood supply to cochlea, whether due to micro-vascular or macro-vascular compromise, can lead to capillary constriction within the stria vascularis, cell death and poorer hearing sensitivity, also the free radicals and Reactive Oxygen Species (ROS) generated during the metabolism of excess triglyceride would increase the necrosis/apoptosis of inner ear cells and lead to hearing loss [12].
2. Aim of the Study
- Aim of the current study was to evaluate the relationship between obesity and hearing in a group of Egyptian obese children.
3. Material and Methods
3.1. Participants
- Eighty children of both genders were enrolled in this study and divided into two groups.Study group: Forty (40) healthy obese children were randomly selected from the outpatient pediatric clinic.Control group: Forty (40) non-overweight children age and sex matched serve as controls. Design: The study was carried out in Alzhraa university hospital – Faculty of Medicine for girls – Alazhar University, Cairo Egypt: audiological, pediatric and biochemistry departments.Participants were evaluated during a hospital visit to determine family medical history, current medical conditions, medication use, self-report of the presence of any smokers in the household, socioeconomic and demographic information.In addition, physical examinations, measurements; including height, weight, waist and hip circumference and laboratory test using blood samples for lipid profile and serum insulin. Children with the following were excluded from this study:Ÿ History of chronic exposure to noise. Ÿ History of short- term exposure to loud noise (e. g. Explosions, firearm noise). Ÿ History of ear discharge, perforated tympanic membrane or any other chronic ear disease. Ÿ Diabetes and hypertension. Ÿ Organic or psychiatric illness. Ÿ History of trauma to the ear. Ÿ Family history of hearing loss. Ÿ On medication [ototoxic drugs, sedatives within last 2 months]. Ÿ Smokers.
3.2. Anthropometric Measurements
- Each subject’s height, weight, and waist circumference (WC) were measured after overnight fasting. BMI was calculated by dividing body weight in kilograms by squared height in meters (kg/m2). The body weight was measured using Seca scale closest to 0.1 kg in barefoot and light dress, after emptying the urinary and gastrointestinal apparatuses (Seca Model 770, Hamburg, Germany). BMI in children is compared to age and sex-specific reference values. In this study, the definitions of “obesity” were based on recommendations by the Centers for Disease Control and Prevention Subjects with a BMI ≥ 95th percentile were classified as obese [13]. Underweight subjects were excluded from the analyses.The waist circumstance (WC) was determined at the point midway between the superior border of the iliac crest and lower border of the rib cage using non-elastic tape nearest to 0.5 cm at the normal expiration, and their hip circumstance (HC) was measured to the nearest 0.5 cm at the widest part of the hip at the levels of the greatest trochanter by a non-stretchable tape meter. The waist to hip ratio (WHR) was computed from dividing WC by HC [15].
3.3. Audiometric Measures
- All subjects included were subjected to the following: Otological examination, Audiometric testing using Piano plus pure tone audiometry (PTA) was done at frequencies 250, 500, 1000, 2000, 4000 and 8000 Hz for air conduction threshold. Bone conduction was tested at frequencies 500, 1000, 2000, 4000 Hz. Immittancemetry were performed using Miaco Mi 44 and Acoustic reflexes (AR) on 500, 1000, 2000 and 4000 Hz ipsilaterally, were performed to insure normal middle ear function. TEOAEs uses Madsen Capella were elicited using non-linear click stimuli at stimulus intensity ranges from 80 dB peak equivalent sound pressure level (SPL), 80μs duration, at a rate of 50 clicks per second, within a time window of 20 msec. TEOAEs were analyzed by recording 260 sweeps in one session and averaged within 5 frequency bands centered at (1, 1.5, 2, 3 and 4 kHz). An acceptable TEOAE is 3 dB above the noise floor (SNR) and the whole reproducibility% was ≥50% according to.
3.4. Sampling
- 1- Serum total cholesterol (TC) and TG concentrations were determined by enzymatic colorimetric tests (CHOD-PAP and GPO-PAP methods, respectively; Roche). High-density lipoprotein cholesterol and LDL cholesterol (LDL-C) levels were measured using enzymatic colorimetric tests after selective separation of high-density lipoprotein and LDL fractions (Direct HDL Cholesterol and Direct LDL Cholesterol; Roche).2- Serum insulin (Test principle):Immunospec Insulin Quantitative Test Kit is based on a solid phase enzyme-linked immunosorbent assay (ENZ-KIT141-0001). The assay system utilizes one anti-Insulin antibody for solid phase (microtiter wells) immobilization and another anti-Insulin antibody in the antibody-enzyme (horseradish peroxidase) conjugate solution. The standards and test specimen (serum) were added to the Insulin antibody coated microtiter wells. Then the anti-Insulin antibody labeled with horseradish peroxidase (conjugate) is added. If human Insulin was present in the specimen, it would combine with the antibody on the well and the enzyme conjugate resulting in the Insulin molecules being sandwiched between the solid phase and enzyme-linked antibodies. After 1 hour incubation at room temperature, the wells are washed with water to remove unbound labeled antibodies.A solution of TMB was added and incubated for 20 minutes, resulting in the development of a blue color. The color development is stopped with the addition of stop solution. The color was changed to yellow and measured spectrophotometrically at 450 nm. The concentration of Insulin is directly proportional to the color intensity of the test sample. The ranges used as guidelines are: Children < 12 yrs < 10 μ IU/ml. Adult (Normal) 0.7 – 9 μ IU/ml. Diabetic (Type II) 0.7 – 25 μ IU/ml. HOMA-IR= Fasting insulin (µu/ml) x fasting glucose (nmol/l) / 22.5 [6].
3.5. Ethical Consideration
- The study protocol was approved by local Ethics Committee and all procedures were in accordance with the Helsinki Declaration. The research was fully explained to all the participants, and an informed consent was obtained from each participant family, participant were told that they were free to withdraw from the research whenever they wished.
4. Statistics
- Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 20. Statistical Analysis Software. The statistical tools used include – Mean, Standard Deviation and T test.
5. Results
- Demographic data and anthropometry of the studied groups revealing that there was significant increase in weight, BMI, neck & waist circumference and waist/hip in the obese group in comparison to controls (table 1).Comparison between the studied groups regarding liver enzymes, lipid profile, fasting serum insulin and HOMA index, showing that there was significant increase in serum AST, triglyceride, fasting insulin and HOMA index in obese group in comparison to controls (table 2).Significant increase of the pure tone threshold in the frequencies (250-8000 Hz) in group II than group I and control in both right and left ear. Increase of the pure tone threshold in the frequencies (250-4000Hz) in the group I than the control but not statistically significant (table 3).Comparison between controls and the group I regard otoacoustic emissions, showing that there was no significant difference between the group I (normal middle ear) and the control regarding otoacoustic emission (table 4).Correlation between pure tone with the waist/hip ratio, BMI and Neck circumference in group II, showing that there was a positive correlation between pure tone threshold and the previous measurements (table 5). Correlation between pure tone with the liver enzyme, triglyceride, cholesterol, fasting insulin and HOMA index in group II, showing that there was a positive correlation between them (table 6).Correlation between pure tone with the liver enzyme, cholesterol, triglyceride, fasting insulin and HOMA index in group I, showing that there was a positive correlation between them (table 7).Correlation between otoacoustic emission with the liver enzyme and lipid profile in group I, showing that there was a negative correlation with liver enzyme, cholesterol, triglyceride, LDL and positive correlation with HDL (table 8).Correlation between otoacoustic emission with fasting insulin and HOMA index (Group I), showing that there was a negative correlation between them (table 9).Correlation between otoacoustic emission with hip / waist ratio, BMI and neck circumference (Group I). Showing that there was a negative correlation between them (table 10).
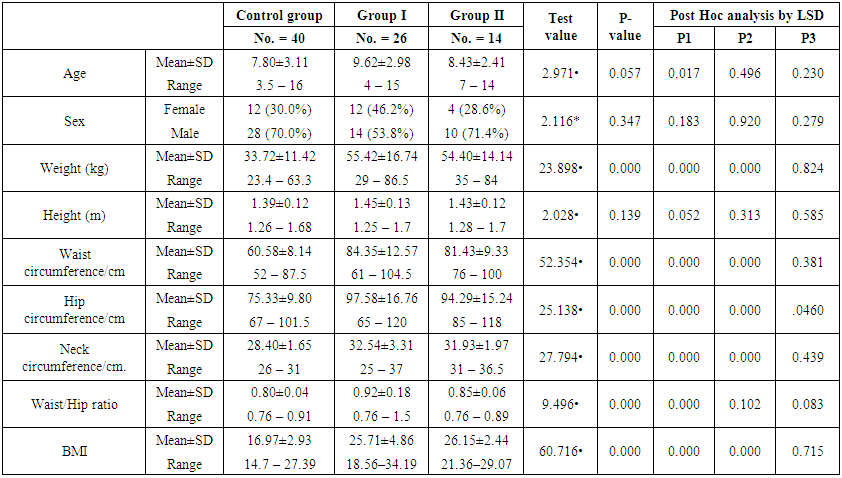 | Table (1). Demographic data and anthropometry in the studied groups |
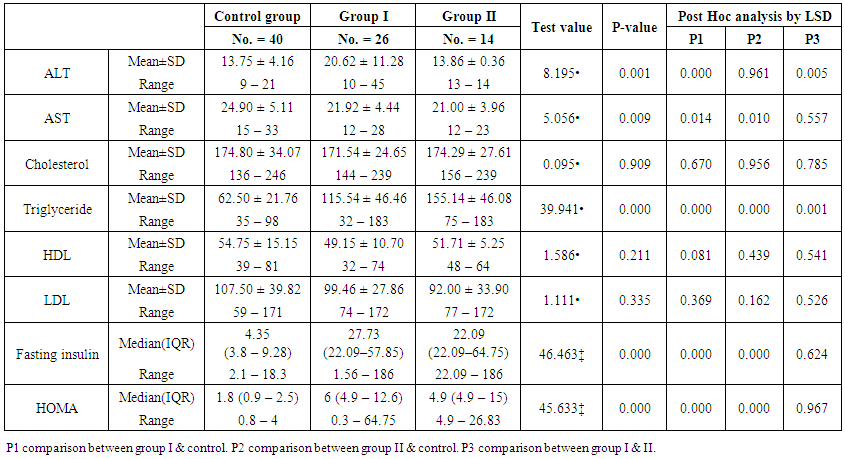 | Table (2). Comparison between the studied groups regarding liver enzymes, lipid profile, fasting serum insulin and HOMA index |
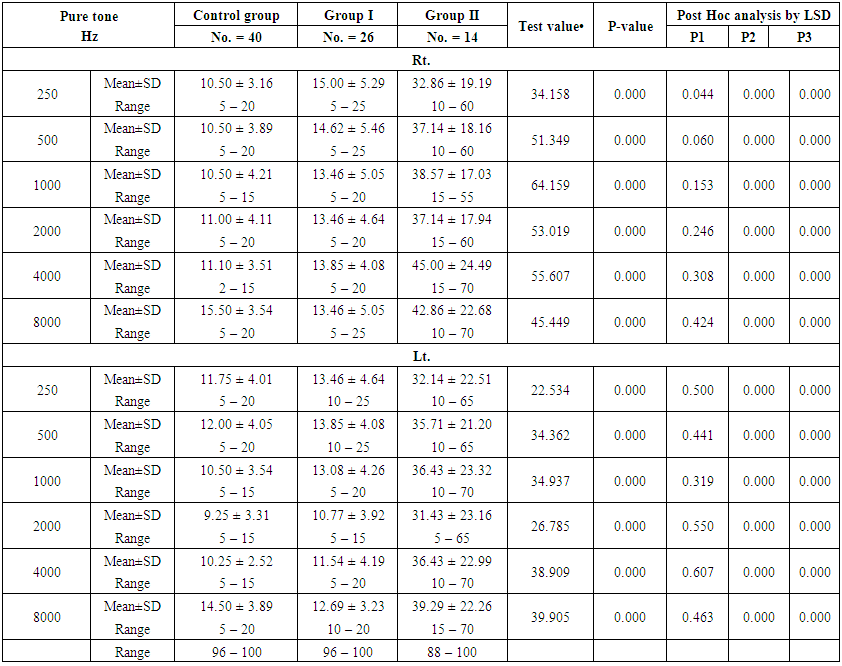 | Table (3). Comparison between the studied groups regarding the pure tone |
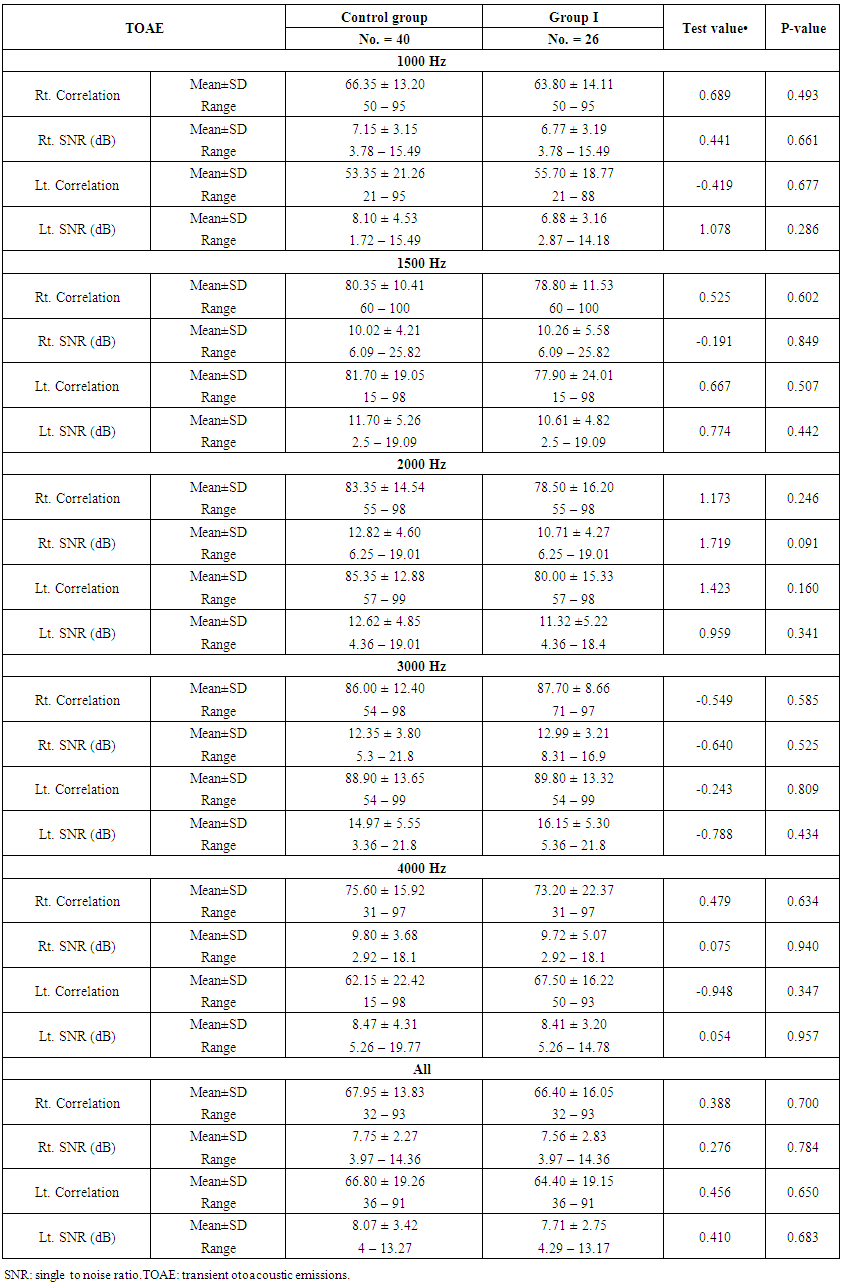 | Table (4). Comparison between group I and the control regarding transient otoacoustic emissions |
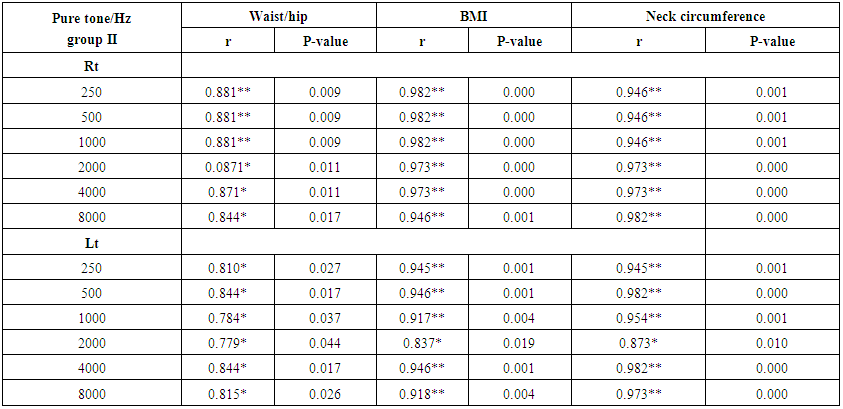 | Table (5). Correlation between pure tone with waist/hip ratio, BMI and Neck circumference in group II |
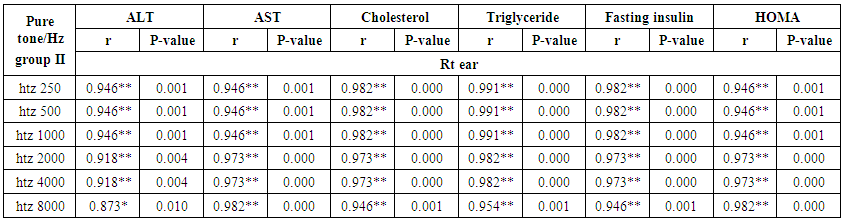 | Table (6). Correlation between pure tone with liver enzyme, triglyceride, cholesterol, fasting insulin and HOMA index in group II |
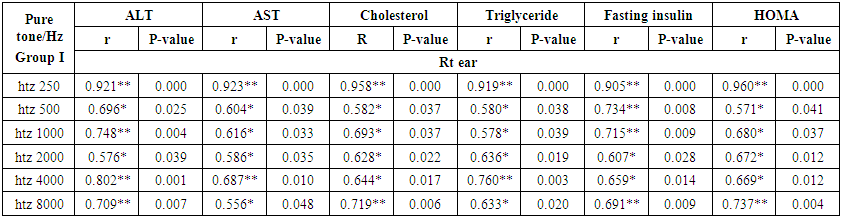 | Table (7). Correlation between pure tone with liver enzyme, cholesterol, triglyceride, fasting insulin and HOMA index in group I |
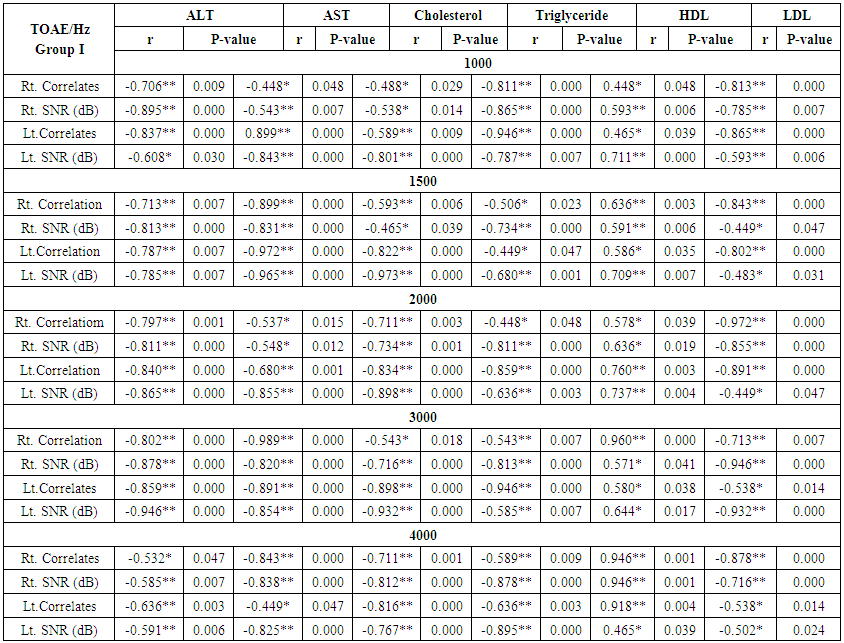 | Table (8). Correlation between otoacoustic emission with liver enzyme and lipid profile in group I |
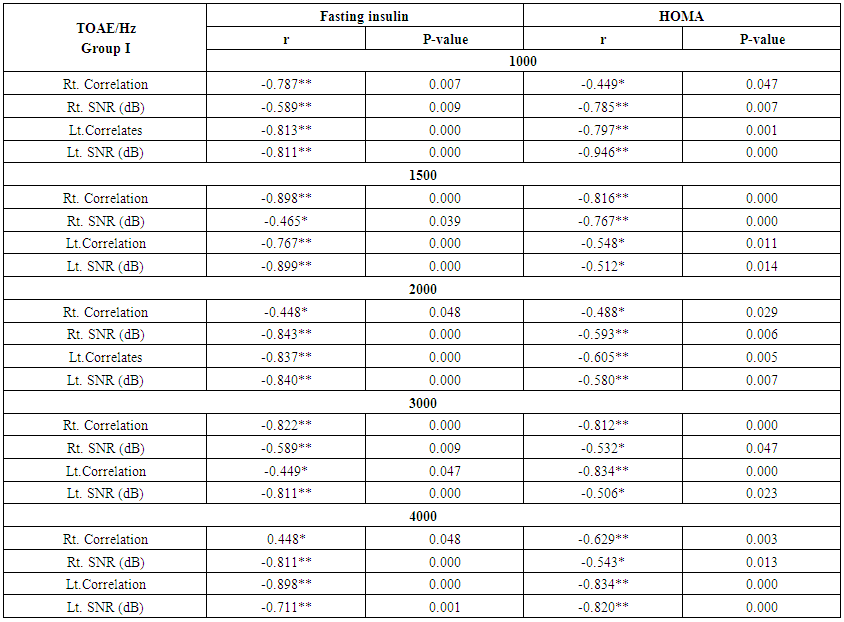 | Table (9). Correlation between otoacoustic emission with fasting Insulin and HOMA index in group I |
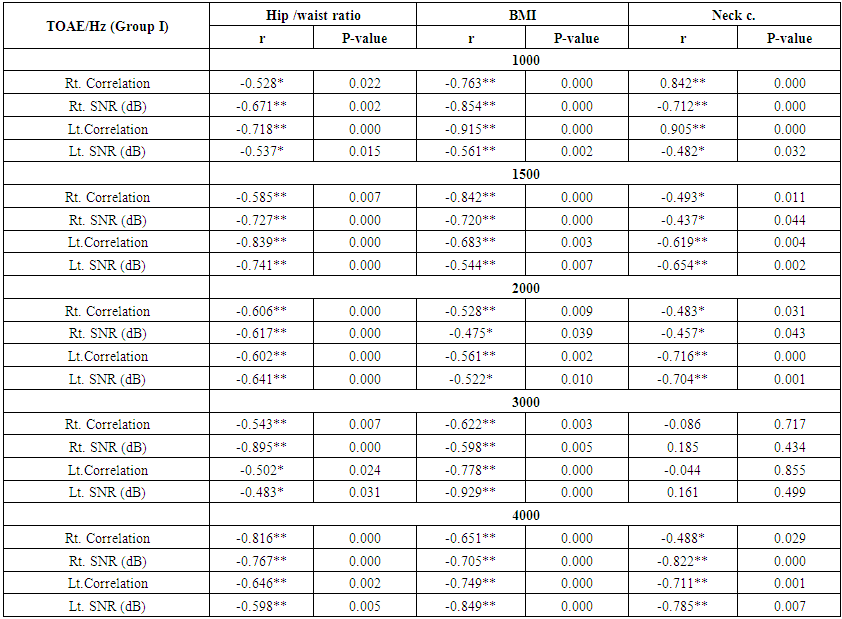 | Table (10). Correlation between otoacoustic emission with hip /waist ratio, BMI and neck circumference (Group I) |
6. Discussion
- The present study was a controlled study that was carried out on 80 children of both genders and divided into two groups. Study group, 40 healthy obese children were randomly selected from the outpatient pediatric clinic where they subdivided after the study has been completed into; group I, twenty- six healthy obese children, 12(46.2%) female and 14(53.8%) males their ages ranging from 4-15 years with normal middle ear function. Group II fourteen obese children, 4(28.6%) female and 10(71.4%) males their ages ranging from 7-14 years with middle ear disease. Control group, included forty lean, non-overweight children 12(30.0%) female and 28(70.0%) males with ages ranging from 3.5 to 16 years, both group I obese children and the control group have normal tympanometry finding while group II tympanometry show that eight of them having eustachian tube dysfunction while six suffering from otitis media with effusion. The aim of the current work was to assess the relationship between obesity and its comorbidities and hearing in a group of Egyptian obese children.In our study there was significant increase in weight, BMI, neck & waist circumference and waist/hip in the obese group in comparison to controls. Mushtaq et al [19] and Kandeel et al [20], they found the same results, also there was significant increase in serum triglyceride, fasting insulin and HOMA index in obese group in comparison to controls. Rizzo et al [21], Juárez-López et al [22] observed in their studies that 56% of the obese children suffered from hyperinsulinemia. Also Romualdo et al [23] observed alterations in fasting insulin and insulin resistance among obese girls.The middle ear comprises the tympanic cavity, consisting of the tympanic membrane, ossicular chain with its muscles and ligaments, the auditory tube, the aditus, antrum and mastoid cells. The three main functions of the auditory tube are: middle ear ventilation, protection and drainage. The function of the auditory tube plays an important role in the etiology of otitis. Otitis media characterized by an inflammation of the middle ear that may be accompanied by an outpouring of fluid accumulated in the middle ear and it is a very common infectious disease in childhood [24]. Otitis media with effusion is one of the most common diseases in children, affecting 28-38% of the pre-school population. Auditory tube dysfunction can cause pathological changes in the middle ear, which in turn can lead to conductive hearing loss or other complications of otitis media. Hearing loss is classified as conductive (referring to lesions on the external and middle ear), sensorineural (lesions of the cochlea or involving the eighth nerve) or mixed [25].In our result the mean hearing threshold in pure tone study of obese children (group II) were significantly higher as compared to their control with respect to all frequencies. Increase in the mean hearing threshold of the pure tone study in the obese children (group I) compared to their control but not statistically significant and no significant difference between the group I (normal middle ear) and the control regarding transient otoacoustic emission.In agreement with our results, Kim et al [26] in study of relationship between pediatric obesity and otitis media with effusion in obese children, they found that the incidence of OME was high in obese group and they suggests that childhood obesity could have an effect on the development of OME.Another study by Kim et al [27], they found that the frequency of patients diagnosed with OME and undergoing ventilation tube insertion was significantly higher in the obese children, but significantly lower in the underweight, normal and overweight children, also Kaya et al [28] were found that the prevalence of overweight or obesity was higher in children with chronic otitis media with effusion. Al-Shawi et al [29] on the study of effects of obesity on the eustachian tube function in Saudi adults, they found that there is no significant difference between adult obese and non-obese patients in middle ear baseline pressure, effects of Valsalva and Toynbee maneuvers or the duration of the effects.Lalwani et al [30] in study of hearing affection in the adolescent aged 12 to 19 years, they found that obesity in adolescents was associated with elevated pure tone hearing thresholds and greater prevalence of unilateral low-frequency sensorineural hearing loss.In our study there was positive correlation between pure tone with the waist/hip ratio, BMI and neck circumference, also there was positive correlation between pure tone with the liver enzyme, triglyceride and cholesterol in the group II and I.In our study there was negative correlation between otoacoustic emission with the liver enzyme, cholesterol, triglyceride and LDL and positive correlation with HDL in group I both on high and mid frequencies. Specifically related to the cochlea, the lipid composition, fluidity, and stiffness of the outer hair cell lateral wall membrane have been shown to be important to its electromotile function and the cochlear amplifier. The lateral wall plasma membrane of the outer hair cell also seems to have less cholesterol than other cells. These data suggest that outer hair cell function may be particularly sensitive to dyslipidemic states. Histologic changes in the guinea pig cochlea in response to dyslipidemia have been identified in the strial marginal layer and in outer hair cells. Hypercholesterolemia may also decrease cochlear vascularity and cause hearing loss. Also, the other proposed mechanism is increased blood viscosity and atherosclerosis of the cochlear vessels due to dyslipidemia which lead to blood perfusion of the cochlea and propagate hearing impairment [31].Shashikala and Srinivas [12] in their study, have found that higher BMI was associated with higher hearing thresholds particularly for lower frequencies. There was a mild degree (26-40dB) of hearing loss in obese group for lower frequencies and they demonstrates that young adults with a BMI of 25 or more are at increasing risk of mild sensorineural hearing loss [31].Gates et al [32], they also reported an inverse relationship between high-density lipoprotein (HDL) levels and hearing thresholds in adult study, suggesting a protective effect of HDL on hearing thresholds.Bainbridge et al [33], in study of risk factors for hearing impairment among U.S. adults with diabetes show that the prevalence of low HDL was significantly greater in those who were hearing-impaired at the low/mid-frequency range, whereas the prevalence of high total cholesterol was significantly higher in those who were hearing-impaired at the high-frequency range.In our study there was a positive correlation between pure tone with the fasting insulin and HOMA index in group I and II and negative correlation between otoacoustic emission with the fasting insulin and HOMA index in group I both on high and mid frequencies.Koçyiğit et al [34] in study of association between endocrine diseases and serous otitis media in children, the study was conducted on 918 pediatric patients 440 boys, 478 girls; mean age: 8.40, range 3-15 years, they were found up to 55.6% of the children with otitis media with effusion suffering from metabolic syndrome. Seo et al [35] show that impaired fasting glucose and increased HOMA–IR were independent risk factors for high‐frequency mild hearing impairment in the population without diabetes in study of association of hearing impairment with insulin resistance, β–cell dysfunction and impaired fasting glucose before onset of diabetes in adult above age of 20 and below 70 years.Angeli et al [36] in study of alterations in cochlear function during induced acute hyperinsulinemia in an animal model, suggest that acute induced hyperinsulinism suppresses cochlear function.Hyperinsulinemia is an early change in peripheral resistance to insulin. Although classified as a mild disorder, it may significantly affect the inner ear metabolism and fluid concentrations. The negative impact of hyperinsulinemia on inner ear homeostasis is probably due to the ionic and metabolic features of the vascular stria, which is responsible for maintaining the endocochlear potential by secreting potassium into the endolymphatic space. The inner ear is not able to store much energy. Paradoxically, it has a high metabolic activity, especially for maintaining endolymph ionic concentrations [37].Hyperinsulinemia may acts directly on auditory nerve fibers, compromising the ionic transport across the cell membrane and interrupting depolarization. Also acts on the vascular stria, compromising the maintenance of the endocochlear potential, thus interruption hair cell depolarization; this in turn affects subsequent depolarization of neural fiber cells [36].Mangabeira-Albernaz [38] and Fukuda have suggested that hyperinsulinemia could block (Na+/K+) ATPase enzyme activity on the vascular stria, removing potassium from endolymph, retaining sodium, and thus increasing the osmotic pressure at this level.Another explanation to this correlation during the processing of protein oxidation and lipid peroxidation that occur in metabolic syndrome, a large amount of free radicals and Reactive Oxygen Species (ROS) are generated. The free radicals and ROS then cause mitochondria dysfunction and mitochondrial DNA (mtDNA) impairments and lead to cellular degeneration. In the inner ear, the result of this process causes hearing impairment. Another aspect of insulin resistance is impaired insulin signaling in the tissue. Impaired insulin metabolic signaling results in; impaired glucose uptake, endothelial dysfunction, impaired angiogenesis, metabolic inflexibility, vascular stiffness and atherosclerosis. The blood flow is disrupted, causing ischemic events of the cochlea that lead to hair cell apoptosis or necrosis, ultimately resulting in hearing loss [39].
7. Conclusions
- From our study we concluded that, obesity considered as a risk for conductive hearing loss and cochlear affection. Simple anthropometric measurements as BMI, waist/hip ratio and neck circumference can be used as a predictor of hearing impairment in obese children.
8. Recommendations
- Study on large scale of obese children.Extended high frequency audiometry.Screening of hearing and middle ear function for obese children.
ACKNOWLEDGEMENTS
- This work was supported by pediatric and audiology department of faculty of medicine for girl-Alazhar University. We thank the families of children who gave their time in participating in this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML