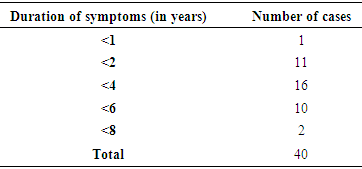-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2018; 7(2): 36-42
doi:10.5923/j.otolaryn.20180702.03

Role of IgE and Absolute Eosinophil Count as Prognostic Markers to Determine the Optimum Duration of Therapy in the Management of Seasonal Allergic Rhinitis
Trisha Srivastava, Karthik Shamanna, Borligegowda Viswanatha
Otorhinolaryngology Department, Bangalore Medical College & Research Institute, Bangalore, India
Correspondence to: Borligegowda Viswanatha, Otorhinolaryngology Department, Bangalore Medical College & Research Institute, Bangalore, India.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

As societies are adapting to western lifestyles the prevalence of allergic diseases such as asthma and allergic rhinitis has increased to epidemic proportions. [1, 2] Conventional drug therapy is used to treat patients with allergic rhinitis according to the ARIA (Allergic rhinitis and impact on asthma) guidelines. [3] However, there are no set parameters or prognostic markers to determine the optimum duration of therapy for long lasting remission of the symptoms. Hence definite parameters or indicators are required to determine the duration of therapy. The aim is to establish the role Serum IgE and AEC as prognostic markers to optimize the duration of therapy in management of allergic rhinitis. Methods: In our study 80 patients with allergic rhinitis were included. They were classified according to their symptoms into categories of mild, moderate, severe allergic rhinitis and treated according to the step wise therapeutic approach of ARIA guidelines. Pre and post therapeutic levels of serum IgE and AEC were evaluated. The severity of symptoms were measured using Total 5 symptom score at the end of 4th week, 8th week, 12th week, 16th week and 6th month. Results: The mean value of serum IgE for the study population pre therapeutically was 322.21 IU/ml which reduced to 104.72 IU/ml post therapeutically with a p value of <0.000178. The mean value of serum AEC for the study population pre therapeutically was 475.9 cells/mm3 which reduced post therapeutically to 292.77 cells/mm3 with a p value of <0.00052. Conclusion: Thus, from our study we conclude that serum IgE and AEC are durable markers to diagnose allergic rhinitis. For the attainment of long term remission of symptoms values of serum IgE and AEC post therapeutically should be within the normal range. The study has to be done on large population to know the positive correlation of symptoms with the levels of serum IgE and AEC and to standardize the values of Serum IgE and AEC to optimize the duration of therapy for long term remission of symptoms.
Keywords: Allergic rhinitis, Serum IgE, AEC
Cite this paper: Trisha Srivastava, Karthik Shamanna, Borligegowda Viswanatha, Role of IgE and Absolute Eosinophil Count as Prognostic Markers to Determine the Optimum Duration of Therapy in the Management of Seasonal Allergic Rhinitis, Research in Otolaryngology, Vol. 7 No. 2, 2018, pp. 36-42. doi: 10.5923/j.otolaryn.20180702.03.
Article Outline
1. Introduction
- Rhinitis is defined clinically by a combination of two or more nasal symptoms: running, blocking, itching and sneezing. Allergic rhinitis occurs when these symptoms are the result of IgE- mediated inflammation following exposure to allergen. [4]The recent Allergic Rhinits and Impact on Asthma (ARIA) document has classified allergic rhinitis as: - Intermittent symptoms- < 4 days per week Or < 4 weeks; Persistent symptoms >4 days per week and > 4 weeks which is further subdivided into: - mild(normal sleep, daily activities, work and school and no troublesome symptoms) or moderate (Abnormal sleep, Impairment of daily activities, sport, leisure, problems caused at school or work, troublesome symptoms). [4] As societies are adapting to western lifestyles the prevalence of allergic diseases such as asthma and allergic rhinitis have markedly increased to epidemic proportions. [1, 2, 5] An worldwide estimate of 400 million people suffering from allergic rhinitis has been made about 50% of whom are from developing nations. [5]3.5% of the total population in India is suffering from allergic rhinitis. [6] The prevalence in India according to the socio-economic stratification are 28.6% in upper class, 33.3% in middle, 27.1% in lower class in urban population and 11.1% in rural population. [6, 7] Allergic rhinitis is a common disease that, although benign, leads to significant impairment of quality of life and a large health care expenditure. [7]Conventional drug therapy is used to treat patients with allergic rhinitis according to the ARIA (Allergic rhinitis and impact on asthma) guidelines. [3] According to the guidelines the treatment is provided until resolution of symptoms. However, there are no set parameters or prognostic markers to determine the optimum duration of therapy for long lasting remission of the symptoms. Hence definite parameters or indicators are required to determine the duration of therapy.In our study, analysis of baseline levels of Immunoglobulin E(IgE) and absolute eosinophil count (AEC) before therapy and subsequent serial measurements of the same during the course of therapy will be carried out. The levels of IgE and AEC will be correlated with resolution of symptoms and the duration of the therapy.The aim is to establish the role Serum IgE and AEC as prognostic markers to optimize the duration of therapy in management of allergic rhinitis.
2. Materials and Method
- The study was conducted on the patients of age group between 18 to 60 years and of either sex presenting with Allergic Rhinitis at hospitals attached to Bangalore Medical College and Research Institute, during the study period from November 2014 to October 2016. In this study 80 patients with allergic rhinitis diagnosed by allergic rhinitis questionnaire (ARIA guidelines) were categorized having mild and moderate/severe symptomatology and treated according to the ARIA guidelines.
3. Aims and Objectives of Study
- • To measure the levels of IgE and AEC before therapy.• To measure the levels of IgE and AEC during therapy.To correlate if IgE and AEC level plays an important role as prognostic markers for determining the duration of therapy in patients with seasonal allergic rhinitis. Inclusion Criteria:• Patient with seasonal allergic rhinitis diagnosed by allergic rhinitis questionnaire (ARIA guidelines).• Patients of either sex aged between 18 and 60 years• Patients willing to give written informed consent. Exclusion Criteria:• Patient with chronic rhinosinusitis infection, chronic purulent postnasal drip, rhinitis medicamentosa.• Patients with nasal polyposis.• Bronchial asthma patients.• Patient with prior history of anti allergic treatment at the beginning of the study.• Patients not willing to give informed consent.• <18 years of age or >60 years.• Pregnant and lactating mothers.Patient with allergic rhinitis fulfilling the inclusion/exclusion criteria were enrolled in the study. Demographic data, medical history, concomitant medications, physical examination, clinical examination including recording of vital signs, systemic examination, lab investigations and details of drug prescription by the treating physician were recorded in the study proforma. 80 patients suffering from mild to moderate/ severe allergic rhinitis, were provided with conventional drugs to treat allergic rhinitis as per ARIA guidelines. Follow-up visits was at 4 weeks (visit 2), 8 weeks (visit 3), 12 weeks (visit 4) and 16 weeks (visit 5) after administering the study drugs. The patients were provided with Total 5 symptom score Questionnaire (T5SS) [8] that includes the following symptoms: nasal discharge (rhinorrhea), nasal congestion, itchy nose, sneezing, and itching eyes was used. All symptoms were graded using a score from 0 (absent) to 3 (very troublesome), with total scores ranging from 0-15. The T5SS was provided to the patients before start of therapy and subsequently in every follow up visit to assess the relief of symptoms. Serum IgE and AEC were measured at the start of the treatment and at follow up visits at 12 weeks and 16 weeks.
4. Results
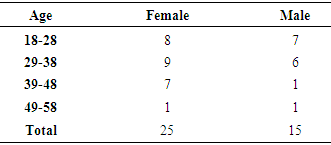
- The age group included in the study ranged from 18-60 years comprising of 80 patients, 50 of which were females and 30 were males. Majority of the allergic rhinitis patients (75%) belonged to the age group of 18–38 years, 37.5% in the age group of 18–28 years and 37.5% in the age group of 29-38 years [Table – 1].
|
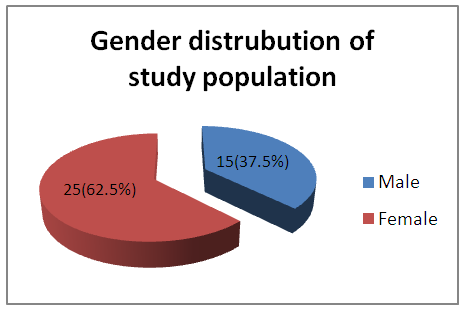 | Graph 1. Gender distribution of study population |
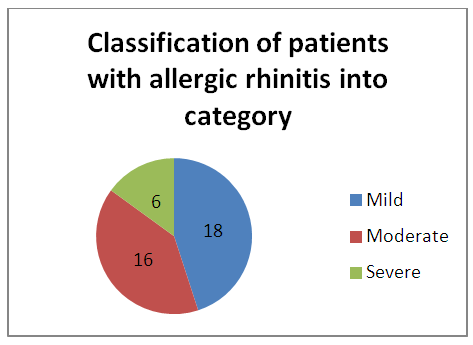 | Graph 2. Classification of patients with allergic rhinitis |
|
|
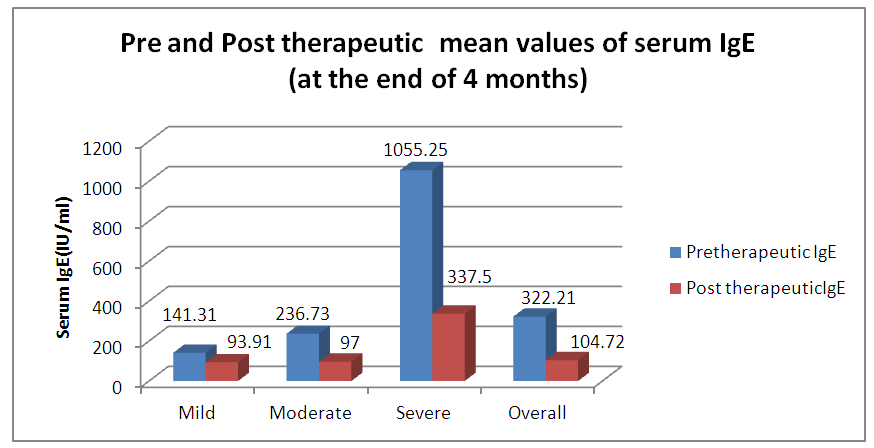 | Graph 3. Pre and Post therapeutic average values of Serum IgE |
|
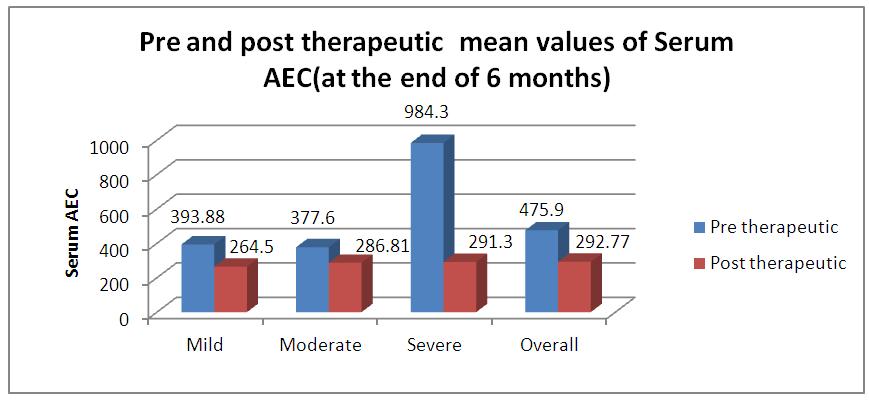 | Graph 4. Pre and Post therapeutic mean values of Serum AEC (at the end of 4 months) |
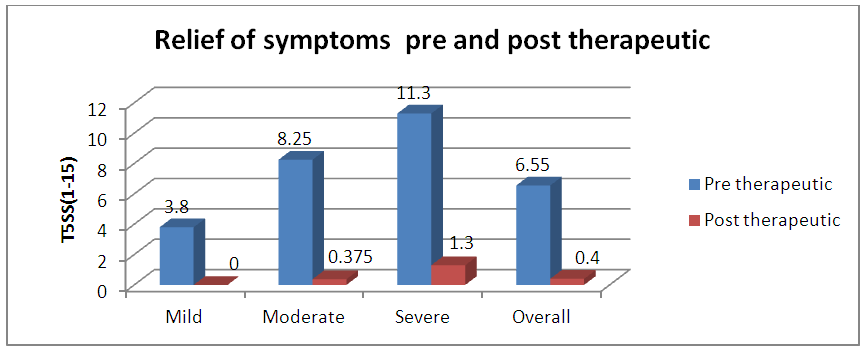 | Graph 5. Pre and Post therapeutic (at the end of 6 months) relief of symptoms using Total 5 symptom score (T5SS) |
5. Discussion
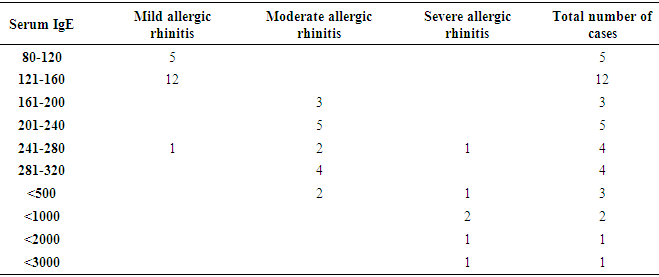
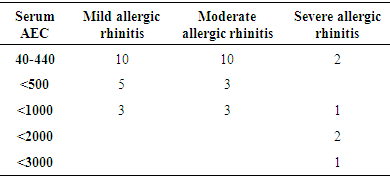
- As per ARIA guidelines a new subdivision of allergic rhinitis has been proposed:- Intermittent (IAR) and Persistent (PER). The severity of allergic rhinitis has been classified as “mild” or “moderate/severe” depending on the severity of symptoms and quality-of-life outcome. Depending on the subdivision and severity of allergic rhinitis, a stepwise therapeutic approach has been proposed. The treatment of allergic rhinitis combines: - Pharmacotherapy, Immunotherapy, Education. [9] However, there are no set parameters or prognostic markers to determine the optimum duration of therapy for long lasting remission of the symptoms.In our study 80 patients with allergic rhinitis were included. The patients with mild symptoms were treated with oral H1-antihistamine or intranasal H1-antihistamine and/or intranasal corticosteroid (CS) or leukotriene receptor antagonist (or cromone), patients with moderate to severe allergic rhinitis were treated with intranasal corticosteroid, H1-antihistamie or leukotriene receptor antagonist (LTRA) which is continued for 4 weeks. If there was improvement then the treatment was stepped down. In case of failure the treatment was modified according to the symptoms. The patients were evaluated using a Total 5 symptom score system which included rhinorrhea, sneezing, nasal congestion, ocular pruritis and nasal pruritis. Each symptom had a score minimum of 1 and maximum of 3 which was graded as mild, moderate, severe symptoms. [8] The scoring was done prior to commencement of therapy and subsequently in the follow up visits at 4th week, 8th week, 12th week, 16th week and at the end of 6 months.Thus, 80 patients with allergic rhinitis were evaluated and studied.The study group comprised of 80 patients between the age group of 18- 60 years. Out of which 50 were females contributing to 62.5% of the sample size, males were 30 in number contributing to 37.5% of the sample size. Majority of the allergic rhinitis patients (75%) belonged to the age group of 18–38 years. The results of the gender and age distribution of our study is in coherence with a clinic Based Cross-Sectional Study from Kolkata conducted by Animesh et al regarding the profile of allergic rhinitis patients, which concluded that, majority of the allergic rhinitis patients (33.3%) belonged to the age group of 30–39 years followed by 30.5% in the age group of 20–29 years. The proportion of females was a bit higher than that of males (57.1% vs. 42.9%). [9]The study population was classified into categories of mild, moderate and severe allergic rhinitis according to their symptoms using the ARIA guidelines. Out of the total 80 patients in the study population 36 patients had (45%) mild symptoms, 32 (40%) patients had moderate symptoms and 12 (15%) patients had severe symptoms of allergic rhinitis. In a clinic based Cross-Sectional Study from Kolkata conducted by Animesh et al the percentage of patients with mild symptoms were 42%, with moderate to severe symptoms were 57.5% which was in coherence with the results obtained in our study. [9]Correlation of Serum IgE and AEC with the severity of symptoms: The value of serum IgE had a positive correlation with the severity of symptoms in patients with allergic rhinitis. The serum IgE value was between 80-160 IU/ml for mild, 161-500 IU/mL for moderate and 700-3000 IU/L for patients with severe allergic rhinitis. The value of serum AEC was within the normal range for 55.5% of patients with mild allergic rhinitis and 62.5% of patients with moderate allergic rhinitis. These patients with serum AEC within the normal range had elevated serum IgE, thus contributing to their symptoms. In the group of patients with severe allergic rhinitis 66.6% of patients had elevated serum AEC levels, suggesting a positive correlation between elevated serum AEC in patients with severe allergic rhinitis.A study conducted by V.S. Chowdary et al compared the value of total Serum IgE and eosinophils in patients with allergic rhintits to that of controls. The serum IgE for allergic patients was found to be in the range of 450IU/ml to that of control which was in the range of 180 IU/ml. The eosinophils in allergic patients was 4% of the peripheral smear to that of 3% in the control group. The levels of serm IgE was found to be in range of 290 IU/ml for mild, 980 IU/ml for moderate and 1201 IU/ml for severe allergic rhinitis. [11]Comparison of pre and post therapeutic serum IgE and AEC: The mean pre therapeutic serum IgE for the study population was 322.21 IU/ml which reduced to 104.72 IU/ml post therapeutically. This reduction in the mean value of serum IgE was statistically significant with a p value of <0.000178. Out of the study sample of 80 patients, 36 patients with mild allergic rhinitis had the mean pre therapeutic serum IgE value of 141.31 IU/ml which reduced post therapeutically to 93.91IU/ml which was not statistically significant. 32 patients of moderate and 12 patients of severe allergic rhinitis had the mean pre therapeutic serum IgE values of 236.73 IU/ml, 1055.25IU/ml respectively which post therapeutically reduced to 97 IU/ml and 337.5IU/ml respectively. The reduction in the serum IgE value for the group having moderate and severe allergic rhinitis was statistically significant with a p value of <0.0048. The mean value of serum AEC for the study group pre therapeutically was 475.9 cells/mm3 which reduced post therapeutically to 292.77 cells/mm3. This reduction in the mean value of serum AEC was statistically significant with a p value of <0.00052. The reduction in the AEC value for the group having severe allergic rhinitis was statistically significant with a p value of <0.005.On comparing the p values of both the factors i.e serum IgE with a p value of < 0.000178 and serum AEC with a p value of <0.00052, serum IgE was a better prognostic marker and correlates well with the severity of symptoms at the time of initial presentation.A study conducted by S.Manohar et al estimated serum IgE level both before and after treatment with histaglobulin and other drugs for 61 patients with allergic rhinitis. Out of 61 patients 55 patients showed reduction in IgE level (90.2%). For 6 patients, the serum IgE level was not reduced. Accordingly the 6 (9.8%) patients did not show clinical improvement. The reduction in IgE level was 80% or above in 5 patients, 50-80% in 11 patients and 15-50% in 30 patients and less than 15% in 9 patients. [12]Hence it can be commemorated that the estimation of serum IgE level is a dependable laboratory test correlating well with the severity of symptoms for patients suffering with allergic rhinitis.The relief of symptoms were assessed using total 5 symptom score pre therapeutically and on the follow up visits of 4th week, 8th week, 12th week, 16th week and at the end of 6th month. The overall improvement in the score was from 6.55 per therapeutically to 0.4 post therapeutic, that was found to be statistically significant with a p value of < 0.05. There was significant reduction in the mean symptom score for patients with mild allergic rhinitis from 3.8 to 0 post therapeutically at the end of 6 months suggesting the 36 patients were completely relieved of their symptoms and had attained remission. The drop in symptom score for moderate allergic rhinitis was from 8.25 to 0.375 i.e. out of the 32 patients, 26 patients had attained complete remission of symptoms, 6 patients were partly relieved of their symptoms and had the value of serum IgE above the normal range post therapeutically. The drop in symptom score for patient with severe allergic rhinitis was from 11.3 to 1.3 post therapeutically at the end of 6 months. Out of the 12 patients with severe allergic rhinitis 4 patients were completely relieved of their symptoms and had attained remission, where as the rest 8 were not completely relieved of their symptoms and post therapeutically had serum IgE above the normal range. The serum AEC of all the patients was in normal range at the end of the treatment except one patient who had severe allergic rhinitis with a pre therapeutic value of 2277 cells/mm3 that dropped to 496 cells/mm3.A study conducted by S.Manohar et al estimated serum IgE level both before and after treatment and its correlation with improvement of symptoms. In this study 61 patients were treated with histaglobulin and other drugs. Out of 61 patients 55 patients showed reduction in IgE level (90.2%). For 6(9.8%) patients, the serum IgE level was not reduced (<100 IU/ml) accordingly did not show remission in symptoms. The reduction in IgE level was 80% or above in 5 patients, 50-80% in 11 patients and 15-50% in 30 patients and less than 15% in 9 patients. [12] The results of the study was in coherence with our study i.e. patients with persistently elevated levels of serum IgE at the end of treatment were not completely relieved of their symptoms.
6. Conclusions
- Our study concludes that it’s advisable to classify patients of allergic rhinitis at the time of presentation for better management. Serum IgE and AEC are reliable markers to diagnose allergic rhinitis. Serum IgE had better correlation with the severity of symptoms for allergic rhinitis.We also conclude that for mild to moderate allergic rhintitis serum IgE is a better prognostic marker then AEC. For the attainment of long term remission of symptoms the values of serum IgE and AEC post therapeutically should be within the normal range.The study has to be done on large population to know the positive correlation of symptoms with the levels of serum IgE and AEC and to standardize the values of Serum IgE and AEC to optimize the duration of therapy for long term remission of symptoms.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML