-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2018; 7(1): 9-14
doi:10.5923/j.otolaryn.20180701.03

Changing Trends in the Management of Malignant Otitis Externa: Our Experience
Karthik Shamanna, Veena B. Ganga
Department of ENT, Bangalore Medical College & RI, Bangalore, India
Correspondence to: Veena B. Ganga, Department of ENT, Bangalore Medical College & RI, Bangalore, India.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A prospective study was conducted at a tertiary referral hospital to evaluate the clinical presentation, co-morbid conditions and treatment response in the management of malignant otitis externa. Among the 34 patients enrolled in the study, 30 were male and 4 were female, aged between 48 to 61 years. Excruciating ear ache was the most common presenting symptom followed by persistent ear discharge and 8 patients presented with facial nerve palsy. 24 patients were diabetic and 11 patients had impaired renal function. Pseudomonas aeruginosa was isolated from 18 patients. All patients were treated conservatively with meticulous aural toilet, intravenous antibiotics and analgesics along with topical eardrops and diluted vinegar ear wash. All patients responded well to medical line of management with emphasis on good glycemic control. Surgical intervention played a less significant role. At follow up, the patients were pain free, with dry ear and good resolution of edema and granulation tissue. 2 patients had residual facial nerve palsy. This study outlines the management protocol of malignant otitis externa at our institute and the changing trends in its treatment are discussed.
Keywords: Malignant Otitis Externa (MOE), Diabetes mellitus, Facial palsy, Pseudomonas aeruginosa
Cite this paper: Karthik Shamanna, Veena B. Ganga, Changing Trends in the Management of Malignant Otitis Externa: Our Experience, Research in Otolaryngology, Vol. 7 No. 1, 2018, pp. 9-14. doi: 10.5923/j.otolaryn.20180701.03.
1. Introduction
- Malignant otitis externa is an aggressive, highly morbid and rarely life threatening infection of the soft tissues of the external ear and surrounding structures, which spreads to involve the periosteum and bone of the skull base [1]. The term malignant otitis externa is a misnomer as it is not a neoplastic condition, but rather the disease spreads and deteriorates rapidly like malignancy, involving normal tissues, especially among elderly diabetic individuals. Hence it is a dreaded condition for any practicing otorhino-laryngologist. The first reported case was published in 1838 by Toulmouche [2] and the term ‘malignant otitis externa’ was first used by Chandler in 1968, because of its high morbidity and mortality [3]. The infection usually originates from the external auditory canal and progresses through the stages of cellulitis, chondritis, periostitis, osteitis and finally osteomyelitis. It is referred to as “skull base osteomyelitis” once the bone infection is confirmed and also known as necrotizing otitis externa due to extensive soft tissue involvement [1]. The disease most commonly affects elderly diabetic and other immune-compromised individuals. The most common organism implicated is Pseudomonas aeruginosa, but organisms like methicillin resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Proteus, Klebsiella are also involved. Aspergillus fumigatus and candida species are the common fungi reported. The most common cranial nerve involved is facial nerve.Malignant otitis externa is basically a clinical diagnosis. However in some cases radiological investigation like high resolution CT of temporal bone, MRI and radio labeled bone scan is helpful in identifying early bone involvement and to rule out complications.The earlier treatment for malignant otitis externa included wide surgical debridement of necrotic tissues and even canal wall down mastoidectomy with facial nerve decompression in most of the cases. However the results were poor and resulted in high morbidity. The results of facial nerve decompression were also not satisfactory. In the present scenario with the advent of highly potent broad spectrum antimicrobial therapy covering drug resistant Pseudomonas and other gram negative pathogens, the management of malignant otitis externa is promising. Our experience with treatment of 34 patients with malignant otitis externa is outlined here.
2. Materials and Methods
- A prospective study was conducted over a period of 3 years (January 2014-2017) at the department of otorhinolaryngology, Bangalore Medical Collage and Research Institute. 34 patients diagnosed with malignant otitis externa during this time period were included in this study. Data regarding the clinical presentations, coexisting disabilities and response to treatment with broad spectrum parenteral antibiotics, steroids and local treatment were analyzed.The objective of the study is to discuss the management of malignant otitis externa in terms of clinical presentation, response to treatment and prognosis at our institute among 34 patients diagnosed and treated for malignant otitis externa.The diagnosis was made on the basis of the patient’s history, clinical examination, microbiology of ear discharge and in a few cases with radiological investigations. All the 34 patients were treated as in-patients in the Otorhinolaryngology department during the study period. All patients had undergone routine blood investigations with special emphasis on diabetic profile, renal status and other immune-compromised state. After obtaining pus from the external auditory canal for culture and sensitivity, patients were started empirically on intravenous Piperacillin and Tazobactam combination (Piptaz) injections of 4.5g strength at Q8H given intravenously as a slow infusion in 100ml normal saline over 15 to 20 minutes. The dose was reduced to half in patients with renal impairment. Based on the patient’s response to therapy along with pus culture and sensitivity report, the injection was continued for 3 weeks. Patients who were not responding after 5 days of Piperacillin and Tazobactam combination injections, were switched over to injection Meropenem, 1gram Q8H given as intravenous infusion in 100ml normal saline over 15 to 20 minutes for a period of 3 weeks. Topical treatment with Beclomethasone and antibiotic (Neosporin or Chloramphenicol) combination ear drops along with 1:1 diluted vinegar ear drops were started. The patients were advised to apply 3 drops thrice in a day. Strict glycemic control was maintained throughout the therapy period with insulin injections. Enzyme preparation (Trypsin, Bromelain and Rutoside Trihydrate) was given orally thrice in a day for a week to reduce the edema of the external auditory canal. 8 patients who had extensive granulation tissues in the external auditory canal received intravenous Dexamethasone (Dexona) injection 4 mg twice a day for 5 days under strict glycemic control. The pain was controlled with paracetamol infusions Q8H. 1:4 diluted vinegar ear wash was given on alternate days throughout the therapy period. Regular microscopic suction clearance was done to evacuate the pus and debris. In patients with elevated blood sugar level, endocrinologist and physician opinion was obtained and good glycemic control with insulin injection was maintained during the entire therapy period. Renal function was regularly monitored by assessment of serum creatinine and blood urea nitrogen. Nephrologist’s opinion was obtained in cases of deranged renal parameters.Facial nerve paralysis was managed with oral prednesolone 60 mg per day in 3 divided doses for 10 days and later tapered to 40 mg in 2 divided doses for another 5 days, further tapering of steroid was carried over 15 days duration (20 mg for 5 days, 10 mg for 5 days and 5 mg for 5 days). Acyclovir tab 400 mg 5 times daily was given orally for one week. Eye protection with overnight upper eyelid taping and hydroxypropyl methylcellulose (Moisol) eye drops was prescribed. Patient underwent regular facial electrical stimulation and physiotherapy.Once the patient showed significant resolution of symptoms, they were discharged home and advised to continue therapy at home. Parenteral antibiotic injection was continued for a week after complete cessation of pain. Topical ear drop and vinegar wash were continued for further 2 weeks. Patients with facial paralysis were advised to continue medications and physiotherapy at home. Strict control of blood sugar was emphasized. All patients were regularly followed up in the outpatient department at twice weekly interval for 2 weeks and later weekly interval for a month.
3. Results
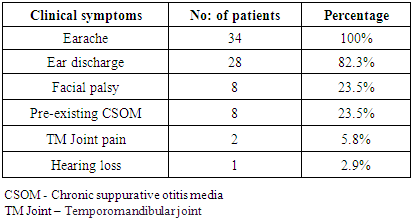
- 34 patients diagnosed with malignant otitis externa were enrolled in this study, 30 were male and 4 were female. The patients were within the age group of 48 to 61years. All the 34 patients had ear ache, which was excruciating, dull, nagging type of pain affecting the external auditory canal, mastoid and the surrounding region. Associated ear discharge was present among 28 patients and pre-existing CSOM in 8 patients (23.5%). 8 patients (23.5%) had facial nerve palsy ranging from grade 3 to 4 according to the House Brackmann scoring system. Among the 8 patients, 1 patient subsequently developed facial nerve palsy with features of malignant otitis externa bilaterally. 2 patients presented with swelling over the TM joint associated with tenderness and restricted mouth opening. The clinical presentations of these patients are represented in table 1.
|
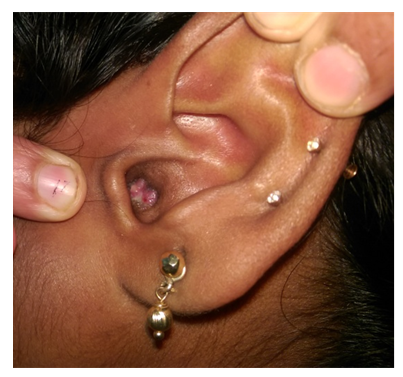 | Figure 1. Granulation tissue in external auditory canal |
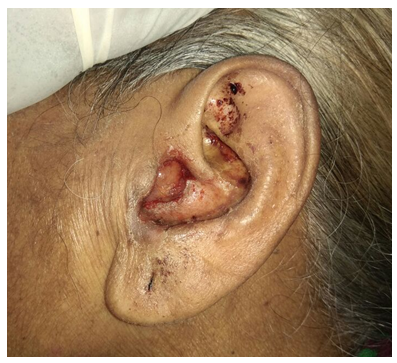 | Figure 2. Aural polyp |
|
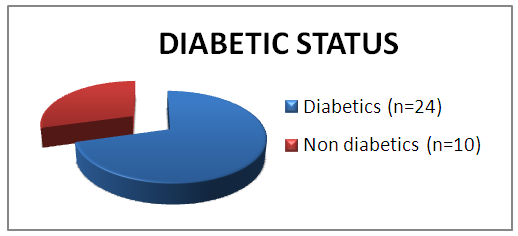 | Graph 1. Diabetic profile of patients |
 | Figure 3. Granulation in left EAC |
|
|
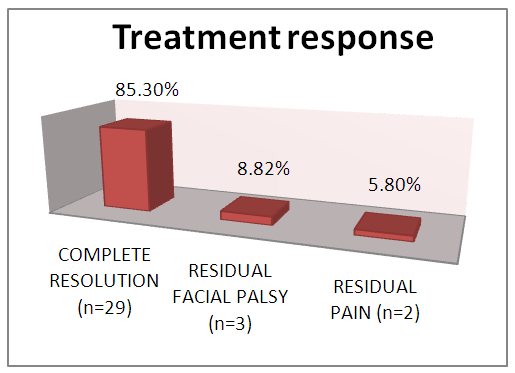 | Graph 2. Treatment response |
4. Discussion
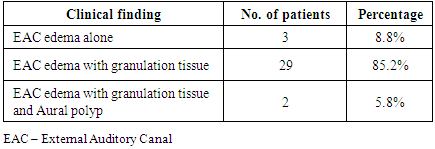
- Malignant otitis externa is an aggressive form of infection affecting the external auditory canal, surrounding soft tissue and bone. It is a rapidly progressing disease occurring most commonly in immunocompromised patients especially uncontrolled diabetics. Levenson’s criteria for diagnosing MOE includes: refractory otitis externa, severe nocturnal otalgia, purulent otorrhoea, granulation tissue in the external auditory canal, growth of Pseudomonas aeruginosa from the external auditory canal and the presence of diabetes or immune-compromised state [4, 5]. Clinico-pathological classification system has divided the disease process into 4 stages [1].Stage 1: Clinical evidence of malignant otitis externa with infection of soft tissues beyond the external auditory canal, but negative Tc-99 bone scan.Stage 2: Soft tissue infection extending beyond the external auditory canal with a positive Tc-99 bone scan.Stage 3: As above, but with cranial nerve paralysis
 Stage 3a: Single cranial nerve palsy.
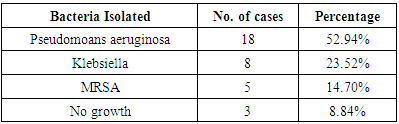
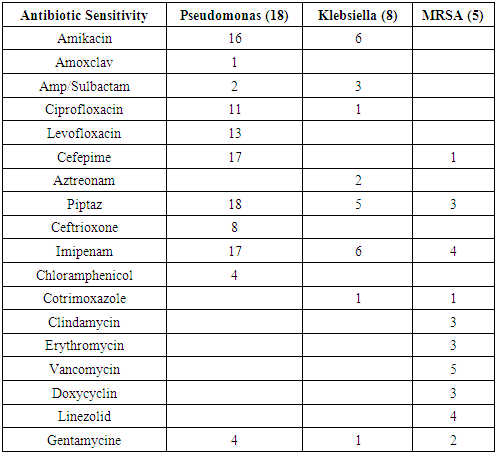
Stage 3a: Single cranial nerve palsy. Stage 3b: Multiple cranial nerve palsy.Stage 4: Meningitis, empyema, sinus thrombosis or brain abscess.The most common presenting symptom in our study was severe excruciating earache which was out of proportion to clinical features, followed by granulation tissue in the EAC, ear discharge, facial asymmetry, TM joint pain and hearing loss. Malignant otitis externa spreads rapidly around the skin and soft tissues of the external auditory canal, leading on to the involvement of periosteum which could be the reason for the severity of earache in all patients. It later spread through the haversian canals of the compact bone producing multiple abscesses and sequestrum. The most common organism isolated in our study was Pseudomonas aeruginosa followed by Klebsiella and methicillin resistant Staphylococcus aureus. Pseudomonas is an obligate gram negative aerobe that tends to colonize in a moist external auditory canal, especially in individuals with impaired immunity. Whereas Staphylococcus, Proteus and Klebsiella are not true pathogens, but they are commensals. Pseudomonas in the absence of an effective immune barrier becomes pathogenic due to the presence of a mucoid layer that is resistant to phagocytosis, it also produces various lytic enzymes that induce necrotizing vasculitis and endarteritis, allowing invasion to the surrounding tissue. High moisture content in the canal predisposes to Pseudomonas infection. Hence the entry of water could be an added factor which predisposes them to infection. Since Pseudomonas is the main causative organism involved, systemic anti-pseudomonal antibiotics are the primary therapy. In the past 15 years, oral fluoroquinolines especially ciprofloxacin had replaced the parenteral therapy. The emergence of quinolone resistant Pseudomonas is the major issue now. Fungal infection constitutes 10% of the incidence. Aspergillus niger, Aspergillus fumigates, Scedosporium apiospermum, Malassezia sympodialis and Candida are the common species isolated [6, 7]. Due to the acute nature of the condition where prompt management had to be initiated at the earliest, fungal and anaerobic bacterial profile was not included in our study. The diagnosis of malignant otitis externa is usually based on clinical presentations and bacteriological study. The role of imaging has often been supportive. High resolution CT scan of temporal bone and MRI of head and neck help to evaluate bony and soft tissue involvement respectively. It is helpful for determining the presence of osteomyelitis, extent of bony involvement, assess the disease status and also in deciding the treatment duration. Radio labeled bone scan is useful in detecting bone involvement in the early stages and it includes (Technetium) Tc-99 bone scan, (Gallium citrate) Ga-67 bone scan and (Indium) In-111 labeled leukocyte scanning [1]. In our study HRCT of temporal bone was done in 26 patients. It was useful in determining the extent of the disease process and presence of any intracranial complications.Emin K et al did a retrospective study over a period of 5 years among 10 patients and concluded that the most effective treatment is to control the diabetic status and to fight against infection with proper antibiotic, debridement and sometimes if required surgical management [8].In our study, 17 patients had uncontrolled diabetes and 7 patients had fairly good diabetic status. 11 patients (32%) had impaired renal functions. The factors which predispose diabetic patients to MOE include impaired phagocytosis, poor leucocyte response, micro-angiopathic disease and endarteritis [9]. Also the cerumen of diabetics has a higher pH (alkaline) which reduces the bactericidal properties of cerumen [10]. Hence addition of acidic (diluted vinegar) ear drops and ear wash might be beneficial in these patients. Most of the patients with malignant otitis externa have diabetes, often associated with other co-morbidities like impaired creatinine clearance. Hence the response to treatment among such immune-compromised patients will also be delayed and both the factors will affect the prognosis of the disease. In spite of all these hindrances, in our study, almost all the patients responded well to parenteral antibiotics with good glycemic control. The facial nerve is the most commonly affected cranial nerve (60 percent of cases with palsies) [5]. The infection usually involves the extratemporal portion of the facial nerve near the stylomastoid foramen. Thereafter, the IX, X and XI cranial nerves are involved as the infection spreads to the jugular foramen. Paralysis of the VI or XII cranial nerve is rare, but has been reported [11, 12]. Halsey et al reported the presence of facial nerve palsy in 75% of patients with Aspergillus infection, compared with only 34% in malignant otitis externa due to pseudomonas [13]. Illing E et al did a case study on malignant otitis externa with skull base osteomyelitis including facial palsy. Patients were treated with parenteral antibiotics followed by canal wall up procedure, following which he observed symptomatic improvement. Treatment of malignant otitis externa and skull base osteomyelitis is a long process. Meticulous aural toilet, antibiotics and strict glucose control in patients with diabetes, are absolutely vital for success. Surgical resection of diseased bone is not recommended due to disease spread through fascial and vascular planes and decompression is not indicated in facial nerve palsy [14].In our study, 23.5% of patients had facial nerve palsy, which was ranging from House Brackmann grade 2 to 4. One patient had bilateral involvement. No other cranial nerve palsies were reported in our study. Majority of patients responded well to additional steroid and supportive therapy. None of the patients underwent any surgical procedures other than routine suction clearance of discharge and debris. Study by Bhat V et al was a retrospective analysis among 15 patients with malignant otitis externa. They concluded that the treatment is mainly medical with appropriate antibiotics. Surgery has a limited role in the treatment and is reserved for cases not responding to medical therapy and also in extensive necrosis. In this study they did surgical treatment for 12 patients for removal of granulation tissue and biopsy [15].The treatment of malignant otitis externa is a therapeutic challenge for the surgeon as there are no specific guidelines for treatment and no prescribed indications for surgical management. During earlier years, malignant otitis media was considered as a very dreaded condition with high morbidity and mortality. Since there was extensive necrosis and uncontrolled spread of infection around the temporal bone, radical surgical debridement was the preferred choice. The outcome of such surgical procedures was limited and some authors even reported that extensive surgical debridement may help in the spread of infections by exposing the healthy bone [14]. However in the present situation, with the advent of highly potent antibiotics against pseudomonas and with better tissue penetration, the trend in management of malignant otitis media has shifted to conservative approach with very good response to parenteral antibiotics, meticulous aural toilet, analgesics, local antibiotic ear drops, diluted vinegar ear wash, along with management of other co-morbid conditions such as maintaining strict glycemic control and managing renal dysfunction. Our study re-emphasizes this changing trend towards conservative management with better outcome and less morbidity. The indication for surgery is limited to local excision of necrotic tissue, draining of abscesses and for taking biopsy to rule out other conditions.
Stage 3b: Multiple cranial nerve palsy.Stage 4: Meningitis, empyema, sinus thrombosis or brain abscess.The most common presenting symptom in our study was severe excruciating earache which was out of proportion to clinical features, followed by granulation tissue in the EAC, ear discharge, facial asymmetry, TM joint pain and hearing loss. Malignant otitis externa spreads rapidly around the skin and soft tissues of the external auditory canal, leading on to the involvement of periosteum which could be the reason for the severity of earache in all patients. It later spread through the haversian canals of the compact bone producing multiple abscesses and sequestrum. The most common organism isolated in our study was Pseudomonas aeruginosa followed by Klebsiella and methicillin resistant Staphylococcus aureus. Pseudomonas is an obligate gram negative aerobe that tends to colonize in a moist external auditory canal, especially in individuals with impaired immunity. Whereas Staphylococcus, Proteus and Klebsiella are not true pathogens, but they are commensals. Pseudomonas in the absence of an effective immune barrier becomes pathogenic due to the presence of a mucoid layer that is resistant to phagocytosis, it also produces various lytic enzymes that induce necrotizing vasculitis and endarteritis, allowing invasion to the surrounding tissue. High moisture content in the canal predisposes to Pseudomonas infection. Hence the entry of water could be an added factor which predisposes them to infection. Since Pseudomonas is the main causative organism involved, systemic anti-pseudomonal antibiotics are the primary therapy. In the past 15 years, oral fluoroquinolines especially ciprofloxacin had replaced the parenteral therapy. The emergence of quinolone resistant Pseudomonas is the major issue now. Fungal infection constitutes 10% of the incidence. Aspergillus niger, Aspergillus fumigates, Scedosporium apiospermum, Malassezia sympodialis and Candida are the common species isolated [6, 7]. Due to the acute nature of the condition where prompt management had to be initiated at the earliest, fungal and anaerobic bacterial profile was not included in our study. The diagnosis of malignant otitis externa is usually based on clinical presentations and bacteriological study. The role of imaging has often been supportive. High resolution CT scan of temporal bone and MRI of head and neck help to evaluate bony and soft tissue involvement respectively. It is helpful for determining the presence of osteomyelitis, extent of bony involvement, assess the disease status and also in deciding the treatment duration. Radio labeled bone scan is useful in detecting bone involvement in the early stages and it includes (Technetium) Tc-99 bone scan, (Gallium citrate) Ga-67 bone scan and (Indium) In-111 labeled leukocyte scanning [1]. In our study HRCT of temporal bone was done in 26 patients. It was useful in determining the extent of the disease process and presence of any intracranial complications.Emin K et al did a retrospective study over a period of 5 years among 10 patients and concluded that the most effective treatment is to control the diabetic status and to fight against infection with proper antibiotic, debridement and sometimes if required surgical management [8].In our study, 17 patients had uncontrolled diabetes and 7 patients had fairly good diabetic status. 11 patients (32%) had impaired renal functions. The factors which predispose diabetic patients to MOE include impaired phagocytosis, poor leucocyte response, micro-angiopathic disease and endarteritis [9]. Also the cerumen of diabetics has a higher pH (alkaline) which reduces the bactericidal properties of cerumen [10]. Hence addition of acidic (diluted vinegar) ear drops and ear wash might be beneficial in these patients. Most of the patients with malignant otitis externa have diabetes, often associated with other co-morbidities like impaired creatinine clearance. Hence the response to treatment among such immune-compromised patients will also be delayed and both the factors will affect the prognosis of the disease. In spite of all these hindrances, in our study, almost all the patients responded well to parenteral antibiotics with good glycemic control. The facial nerve is the most commonly affected cranial nerve (60 percent of cases with palsies) [5]. The infection usually involves the extratemporal portion of the facial nerve near the stylomastoid foramen. Thereafter, the IX, X and XI cranial nerves are involved as the infection spreads to the jugular foramen. Paralysis of the VI or XII cranial nerve is rare, but has been reported [11, 12]. Halsey et al reported the presence of facial nerve palsy in 75% of patients with Aspergillus infection, compared with only 34% in malignant otitis externa due to pseudomonas [13]. Illing E et al did a case study on malignant otitis externa with skull base osteomyelitis including facial palsy. Patients were treated with parenteral antibiotics followed by canal wall up procedure, following which he observed symptomatic improvement. Treatment of malignant otitis externa and skull base osteomyelitis is a long process. Meticulous aural toilet, antibiotics and strict glucose control in patients with diabetes, are absolutely vital for success. Surgical resection of diseased bone is not recommended due to disease spread through fascial and vascular planes and decompression is not indicated in facial nerve palsy [14].In our study, 23.5% of patients had facial nerve palsy, which was ranging from House Brackmann grade 2 to 4. One patient had bilateral involvement. No other cranial nerve palsies were reported in our study. Majority of patients responded well to additional steroid and supportive therapy. None of the patients underwent any surgical procedures other than routine suction clearance of discharge and debris. Study by Bhat V et al was a retrospective analysis among 15 patients with malignant otitis externa. They concluded that the treatment is mainly medical with appropriate antibiotics. Surgery has a limited role in the treatment and is reserved for cases not responding to medical therapy and also in extensive necrosis. In this study they did surgical treatment for 12 patients for removal of granulation tissue and biopsy [15].The treatment of malignant otitis externa is a therapeutic challenge for the surgeon as there are no specific guidelines for treatment and no prescribed indications for surgical management. During earlier years, malignant otitis media was considered as a very dreaded condition with high morbidity and mortality. Since there was extensive necrosis and uncontrolled spread of infection around the temporal bone, radical surgical debridement was the preferred choice. The outcome of such surgical procedures was limited and some authors even reported that extensive surgical debridement may help in the spread of infections by exposing the healthy bone [14]. However in the present situation, with the advent of highly potent antibiotics against pseudomonas and with better tissue penetration, the trend in management of malignant otitis media has shifted to conservative approach with very good response to parenteral antibiotics, meticulous aural toilet, analgesics, local antibiotic ear drops, diluted vinegar ear wash, along with management of other co-morbid conditions such as maintaining strict glycemic control and managing renal dysfunction. Our study re-emphasizes this changing trend towards conservative management with better outcome and less morbidity. The indication for surgery is limited to local excision of necrotic tissue, draining of abscesses and for taking biopsy to rule out other conditions. 5. Conclusions
- Malignant otitis externa is a serious invasive disease affecting mainly the elderly diabetic patients and other immune-compromised people. The most common presentation is severe otolgia with otorrohea followed by facial nerve palsy and in most of the cases symptoms are out of proportion to clinical signs. Pseudomonas aeruginosa is the most common organism isolated and is usually water borne. Parenteral antibiotic therapy with anti-Pseudomonal agents along with local aural cleaning, under strict glycemic control is the most effective treatment modality for the resolution of symptoms. Steroids are beneficial in management of facial weakness and granulation tissue. The role of surgery is limited in the management of malignant otitis externa.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML