-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2017; 6(2): 23-26
doi:10.5923/j.otolaryn.20170602.02

Efficacy of 25% Glucose in Glycerin and Honey in the Management of Primary Atrophic Rhinitis: A Comparative Prospective Study
Shambulinga Killera, Borligegowda Viswanatha, Maliyappanahalli Siddappa Vijayashree
Otorhinolaryngology Department, Bangalore Medical College & Research Institute, Bangalore, India
Correspondence to: Borligegowda Viswanatha, Otorhinolaryngology Department, Bangalore Medical College & Research Institute, Bangalore, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

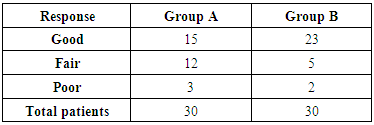
Objectives: To compare the efficacy of 25% glucose in glycerin and honey in the management of Atrophic rhinitis. Materials and Methods: This prospective study carried out between 2006 and 2016, with a total number of 60 patients visiting department of Otolaryngology, Sri Venkateshwara ENT Institute, Bangalore Medical College and Research Institute. The detailed history and clinical examination of each case of atrophic rhinitis was carried out and data were recorded in the preformed case record form. Patients were grouped into two groups according to the treatment plan. In group-A, 25% glucose in glycerin nasal drops was used and in group -B honey was used as a nasal drops. They were followed up for 6 months for the response of the treatment. Result: In group A where 25% glucose and glycerin was used, 50% showed good response with treatment. In group B patients in whom honey was used, 76.66% showed good response. This was statistically significant (P=0.0321). Conclusion: Honey can be used as a substitute for 25% glucose in glycerin nasal drops. It is found to be more effective in relieving the symptoms and signs of the patients.
Keywords: Atrophic rhinitis, Management, 25% glucose in glycerin, Honey
Cite this paper: Shambulinga Killera, Borligegowda Viswanatha, Maliyappanahalli Siddappa Vijayashree, Efficacy of 25% Glucose in Glycerin and Honey in the Management of Primary Atrophic Rhinitis: A Comparative Prospective Study, Research in Otolaryngology, Vol. 6 No. 2, 2017, pp. 23-26. doi: 10.5923/j.otolaryn.20170602.02.
Article Outline
1. Introduction
- Atrophic rhinitis is a chronic debilitating nasal mucosal disease of unknown etiology. It is characterized by progressive nasal mucosal atrophy, with progressive atrophy of the underlying bone of the turbinates, abnormal roomy nasal cavities and formation of viscid secretions and dried crusts leading to a characteristic fetor (ozaena).In pre-antibiotics era, primary atrophic rhinitis was commonly associated with infection with bacteria such as Klebsiella ozaenae. Today, atrophic rhinitis is seen more often occurs as a result of aggressive nasal surgery, trauma, granulomatous diseases, chronic cocaine abuse, and radiation therapy [1]. Sanico and Togias [2] postulated that mucosal changes, along with aging, leads to decreases in function, ability to condition inspired air, and secretion production and results in atrophic rhinitis. The nasal mucosa in atrophic rhinitis gradually changes from a functional, ciliated respiratory epithelium to a nonfunctional lining of nonciliated squamous epithelium, with a loss of mucociliary clearance and neurologic regulation. Crusting, fetor, mucosal atrophy, and widely patent nasal cavities are seen in patients who complain of nasal obstruction [3]. The normal pattern of airflow is changed, which contributes to the sensation of nasal obstruction in addition to decreased olfactory function. This disease was known to the ancient Greek, Indians and Egyptian civilizations. Now a days because of the improved socioeconomic conditions, its incidence in western countries has declined, whereas in Asia, Africa, Eastern Europe, Egypt, Greece, Hungary, Yugoslavia, India, Malaysia and Philippines cases are being reported [4, 5]. Even with the best medical management, patients will continue to have crusting, and may relapse to frank ozaena if maintenance therapy is discontinued. Conservative includes nasal irrigation with a solution prepared with: Sodium bicarbonate – 28.4 g, Sodium biborate – 28.4 g, Sodium chloride – 56.7 g mixed in 280 ml of luke warm water. The crusts may be removed by forceps or suction. 25% glucose in glycerin drops can be applied to the nose thus inhibiting the growth of proteolytic organism. Nasal endoscopes can be used for cleaning the nose.Honey is a natural product available easily. The composition of honey is about 80 % carbohydrate, 18% water and 2% amino acids .Honey has a long medicinal history. The ancient Egyptians not only made offering of honey to gods, they also used it as an embalming fluid and dressing for wounds. Honey has anti bacterial and anti inflammatory properties. Laboratory tests have shown that honey hampers the growth of food borne pathogens such as E-Coli and salmonella, and also effective against staphylococcus aureus and pseudomonas aeruginosa. Along with its antibacterial, it has antioxidant properties also.
2. Materials and Methods
- This study was conducted in 60 patients visiting the outpatient and inpatients department of Sri Venkateshwara ENT institute, Department of Otolaryngology, Bangalore Medical College and Research Institute India, between 2006 and 2016. A total of 60 patients diagnosed with primary strophic rhinitis during this period were distributed randomly in each group.
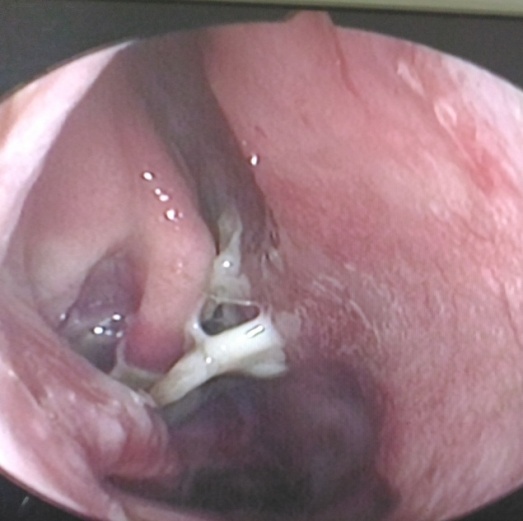 | Figure 1. Endoscopic picture of right nasal cavity shows atrophy of turbinates with foul smelling nasal discharge |
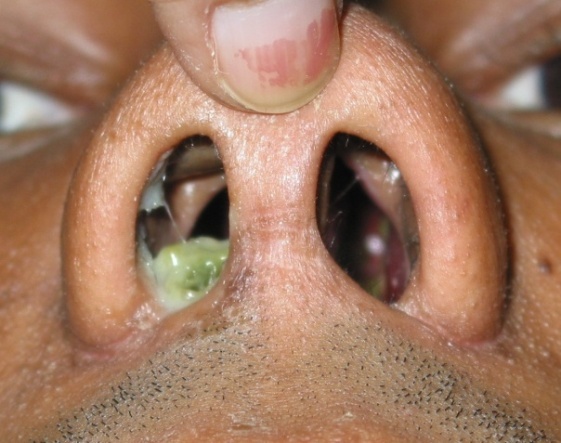 | Figure 2. Photograph showing roomy nasal cavities and greenish crests |
3. Data Analysis
- For the purpose of analysis, all patients were grouped into two, namely Group A and Group B. Data analysis was done with Vassar stats with Chi squared Test.
4. Results
- A total of 60 patients were included in the study for a period of 10 years. In our study out of 60 patients had Primary atrophic rhinitis, peak incidence was between 20 to 30 years with female preponderance. All the patients had bilateral involvement. We observed family history of primary atrophic rhinitis in 4 families. Our patients presented with symptoms of fetor (95%); nasal discharge (60%); nasal obstruction (100%); anosmia (62%); headache (53%); and epistaxis (25%).On clinical examination 89% of them had greenish crusts filling the entire nasal cavities on both sides and 58% also had deviated nasal septum and 18.5% para-nasal sinus tenderness. Three patients had maggots inside the nose. In our study of the 33 patients culture reports revealed growth of Klebsiella ozaenae in 55%, P. areugenosa 17%, Strep. pneumonia 8%, Proteus 4%, mixed growth in 10% and no growth in 6%.In group A where 25% glucose and glycerin was used, 50% showed good response with treatment. In group B patients in whom honey was used, 76.66% showed good response. This was statistically significant (P=0.0321) [table-1].
|
5. Discussion
- Atrophic rhinitis is a disease known since biblical times. It is characterized by progressive atrophy of the nasal mucosa and offensive smell (ozaena). Once the diagnosis of atrophic rhinitis is made, an etiology should be sought. Atrophic rhinitis is divided into two categories: Primary and Secondary. Primary atrophic rhinitis is the classic form of the disease, and its etiology is not known [1]. Factors like heredity, endocrinal disturbance, nutritional deficiency, or autoimmune process are attributed to it. Secondary atrophic rhinitis can be due to syphilis, lupus, leprosy, rhinoscleroma, long standing purulent sinusitis and as a result of nasal surgeries. Atrophic rhinitis is usually seen between the age group of first decade to sixth decade of life and is common in females. In our study patients ranged from 18 to 60 years and peak incidence was between 20 to 30 years with female preponderance. Austin Young [6] whose patients ranged from 8 to 59 years with peak incidence between 10 to 15 years and Males to female ratio was 1:9. Primary atrophic rhinitis is bilateral and unilateral cases are uncommon. In our study all patients had bilateral involvement.Austin Young [6] found a case, inherited the condition from her mother. In a study done by Sinha et al [7], 8.5% of the patients had unilateral disease. Girgis [8] reported family history primary atrophic rhinitis in two patients. Barton et al. [9] proposed that this disease to be dominant in inheritance. In our study, family history of primary atrophic rhinitis was observed in 4 families.Atrophic rhinitis patient’s presents with symptoms of fetor, nasal obstruction, nasal discharge, epistaxis, anosmia which accompanies these degenerative changes. The early sign is the foul smell emanating from the patient. Some patients are psychologically affected at the time of presentation, due to the social implications of the disease. Most prominent finding is nasal crusts filling the entire nasal cavity. Removal of these crusts may induce bleeding. Once the crusts have been removed, several other features may be noted. The mucosa is generally atrophic, with elements of squamous metaplasia present. The nasal cavity may appear roomy due to atrophy of turbinate tissue. Purulent discharge and septal perforations are not uncommon.Our patients presented with symptoms of fetor (95%); nasal discharge (60%); nasal obstruction (100%); anosmia (62%); headache (53%); and epistaxis (25%). On clinical examination 89% of them had greenish crusts filling the entire nasal cavities on both sides. On the contrary, study by Kameswaran et al [10] showed out of the 42 patients, anosmia (100%), nasal discharge (95%) were the commonest symptoms followed by epistaxis and septal perforation, columellar necrosis were also reported along with above findings. One of the interesting finding in our study was the presence of maggots in 4 patients, which due to poor local hygiene as a result of loss of sensation of that area. Various organisms have been cultured from cases of atrophic rhinitis such as Klebsiella ozaenae (Perez bacillus), Diphtheroids, P. vulgaris, Esch. coli, Staphylococci and Streptococci but they are all considered to be secondary invaders responsible for foul smell rather than the primary causative organisms of the disease. In our study of the 33 patients culture reports showed Klebsiella ozaenae in 55% followed by P. areugenosa 17%, Strep. pneumonia 8%, Proteus 4%, mixed growth in 10% and no growth in 6%.The overall therapy encompasses two main goals: restoration of nasal hydration, and minimization of crusting and debris. To achieve these goals, several broad classes of therapies may be used: topical or local, systemic, or surgical. One of the most widely used treatments is nasal irrigation. This can be used with curative intent or as maintenance therapy. Irrigations are used to prevent the formation crusts. Irrigations should be two to three times in a day. As a result, patient compliance is often difficult. Some authors advocate saline douching [3] while others recommend a mixed solution of sodium chloride, sodium bicarbonate and sodium biborate [10] in addition, painting with 25% glucose in glycerin solution or a bland ointment may keep the saprophytic infection down. Systemic or oral therapies are often used in conjunction with the nasal irrigation. The most common type of systemic therapy is antibiotics. Studies have shown that honey is useful in the treatment of chronic wounds and ulcers [11]. Honey has anti bacterial an analgesic effect [12, 13]. Honey stimulates the release of various enzymes like cytokines, tumor necrosis factor and interleukins from monocytes which play a role in healing and tissue repair [14].In our country majority of the patients suffering from atrophic rhinitis were from rural areas. In these areas 25% glucose in glycerin nasal drops is not readily available. Due to this reason patient compliance was found to be less. Honey is a freely available product which is available in rural areas also. Hence patient compliance was found to be satisfactory.
6. Conclusions
- Atrophic rhinitis is an uncommon disorder in many parts of the world. In our country majority of the patients suffering from atrophic rhinitis were from rural areas. In these areas 25% glucose in glycerin nasal drops is not readily available. Due to this reason patient compliance was found to be less. Honey is easily available natural product, which is available in rural areas also. Hence we found that patient compliance was found to be satisfactory. Honey can be used as an adjuvant along with alkaline douche in the management of atrophic rhinitis. Honey is a good substitute for 25% glucose in glycerin nasal drops.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML