-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2016; 5(1): 9-15
doi:10.5923/j.otolaryn.20160501.02

Effects of Cigarette Smoking on Auditory Function
Prem G. Nair1, Jestina J. J.2, Haritha Unnikrishnan1, Haritha Chandrahasan1
1Department of Speech pathology and Audiology, Amrita Institute of Medical Sciences and Research Centre, Amrita Vishwa Vidyapeetham University, Kochi, India
2Institute of Speech and Hearing, Marthoma College of Special Education, Kerala University of Health Sciences, Kasaragod, India
Correspondence to: Prem G. Nair, Department of Speech pathology and Audiology, Amrita Institute of Medical Sciences and Research Centre, Amrita Vishwa Vidyapeetham University, Kochi, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: Few of the studies advocate that cigarette smoking have been associated with development of hearing loss. Both conductive and sensorineural type has been reported. However, comprehensive audiological evaluation on smokers has been scarce and this is especially true with respect to Indian population. Thus there is an extremely important need to assess the hearing status in smokers and to view pathophysiology of auditory system in greater detail. Considering the social relevance of this aspect, particularly in the Indian context, the present study was undertaken. Method:The study consisted of two groups of subjects: the first group (clinical) comprised of 30 males (age range: 15-55 years) who has habit of cigarette smoking and the second group (control) with 30 normal hearing male subjects, without the habit of cigarette smoking, within the same age range. Audiological assessment included: Puretone audiometry including high frequency audiometry, Speech audiometry, Tympanometry, Distortion Product Otoacoustic Emissions (DPOAE) and Brainstem Evoked Response Audiometry (BERA). ResultsandDiscussion: In the present study, pathological auditory involvement was clearly evident in smokers. The puretone audiometric findings indicated reduced hearing sensitivity in smokers. This was particularly evident at high frequency regions as demonstrated by audiogram pattern. Sensorineural hearing loss was more prevalent in smokers. DPOAE findings indicated involvement of cochlear outer hair cells. BERA indicated probable involvement of auditory nerve and brainstem. Conclusions:Periodic audiological evaluation in smokers could demonstrate presence/progress of auditory deficits. Such information may prove effective in helping smokers to quit the habit. Thus audiological evaluation could serve a great social cause.
Keywords: Smoking, Hearing loss, Audiological investigation
Cite this paper: Prem G. Nair, Jestina J. J., Haritha Unnikrishnan, Haritha Chandrahasan, Effects of Cigarette Smoking on Auditory Function, Research in Otolaryngology, Vol. 5 No. 1, 2016, pp. 9-15. doi: 10.5923/j.otolaryn.20160501.02.
Article Outline
1. Introduction
- Tobacco consumption has become a common tendency worldwide and hence tobacco-related diseases are also increasing. Vast amount of data points particularly to the connection between smoking and diseases of the cardiovascular system, lungs and the digestive system [1]. Few of the studies advocate that cigarette smoking have been shown to be highly associated with the development of hearing loss [2], [3].Cotran et al [4] reported that nicotine and other toxic substances contained in cigarettes cause histopathological changes in the respiratory lining tract. Since the mucosa lining the middle ear has the same characteristics of the respiratory tract [5], it is hypothesized that there could be relationship between cigarette smoking and middle ear impairment. Agius et al [6] reported that continued exposure to tobacco may result in persistent middle ear infections and hearing loss. Sharabi et al [1] did a retrospective study on 13,308 smokers (age range of 20-68 years) and explained that smoking can cause mild, flat sensorineural hearing loss (SNHL) or conductive hearing loss. Consumed tobacco may affect cochlear blood supply because it causes peripheral vascular changes, such as increased blood viscosity [7] and reduced available oxygen. Young adult smokers with normal hearing were found to have significantly reduced DPOAEs when compared to their normal counterparts [8], [9]. Further, study done by Gopal et al [10] on normal hearing young male adult cigarette smokers (n=16) revealed that DPOAE and Auditory Brainstem Response (ABR) peak V amplitudes were good predictors for hearing loss. Similarly, Gupta et al [11] reported that ABR abnormalities are observed in smokers (n=40). They noted prolonged latencies of waves I, III, V over left ear and waves III, IV, V over right ear; increased Inter Peak Latencies (IPLs) of I-V, III-V over left ear and of I-III, I-V, III-V over right side. Amplitudes of waves I-Ia and V-Va were decreased bilaterally. These studies indicate the probable impairment of auditory nerve and brainstem level auditory nuclei in signal transmission. Many of the studies conducted on smokers have indicated its negative effects on auditory function. However, comprehensive audiological evaluation on smokers has been scarce and this is especially true with respect to Indian population. Thus there is an extremely important need to assess the hearing status in smokers and to view pathophysiology of auditory system in greater detail. Considering the social relevance of this aspect, particularly in the Indian context, the present study was undertaken.
2. Method
- The study consisted of two groups of subjects: the first group (clinical) comprised of 30 males (age range: 15-55 years) who has habit of cigarette smoking and the second group (control) with 30 normal hearing male subjects, without the habit of cigarette smoking within the same age range (15-55 years). Male subjects smoking cigarettes for a minimum period of five years (minimum five per day) were considered in the clinical group. Both the smokers and the non-smokers were interviewed by using a questionnaire. Subjects with continuous noise exposure for prolonged periods, drug ototoxicity, chronic middle ear infections, hereditary familial sensorineural hearing loss, diabetes mellitus, hyperlipidemia, cerebellopontine angle tumors, head or ear injury, any ear surgery or other neurological/ psychiatric conditions were excluded from the clinical group. All control group subjects had normal hearing, i.e., puretone thresholds 25 decibel (dB) or below at octave frequencies from 250-8000 Hz and including 6 kHz. Subjects with history of occupational noise exposure, ear infections or other neurological/otological/psychiatric conditions, subjects with habit of active smoking or exposure to passive smoking and/or alcohol intake were excluded from the control group. All the subjects participated voluntarily in the study and an informed consent was obtained. The study was carried out in Marthoma College of Special Education, Kasaragod, Kerala, India and the ethics committee of the institute provided ethical approval for the study.Audiological assessment was done to study the effect of cigarette smoking on auditory function which included: Puretone audiometry including high frequency audiometry, Speech audiometry (Speech Reception Threshold-SRT and Speech Discrimination Score-SDS), tympanometry, Distortion Product Otoacoustic Emissions (DPOAE) and Brainstem Evoked Response Audiometry (BERA). GSI-61 clinical audiometer (puretone audiometry), GSI-Tympstar (tympanometry), GSI-Audera (DPOAE) and Intelligent Hearing Systems (IHS-BERA) instruments were utilized.Air conduction thresholds were obtained using puretones at frequencies of 250 Hertz (Hz), 500 Hz, 1 kilo Hz (kHz), 2 kHz, 4 kHz, 6 kHz, 8 kHz and also high frequencies such as 10 kHz, 12.5 kHz, 16 kHz and 20 kHz using modified Hughson and Westlake procedure [12]. Bone conduction thresholds were obtained using puretones at frequencies 250 Hz, 500 Hz, 1 kHz, 2 kHz and 4 kHz. For SRT estimation, spondee words were employed and monosyllables were used for determining SDS. The tympanometry was performed using 226 Hz probe tone. The DPOAE Signal to Noise Ratio estimation was done at frequencies of 598 Hz, 691 Hz, 785 Hz, 902 Hz, 1031 Hz, 1184 Hz, 1371 Hz, 1594 Hz, 1816 Hz, 2098 Hz, 2402 Hz, 2754 Hz, 3152 Hz, 3621 Hz, 4184 Hz, 4816 Hz, 5496 Hz, 6340 Hz and 7277 Hz. For BERA, gold cup electrodes were employed. The results obtained on each of the audiological tests for smokers were compared with the control group separately. The high frequency audiometric thresholds (4 kHz-20 kHz) were separately analyzed between smokers and control group. The data were subjected to statistical analysis using Statistical Package for the Social Sciences (SPSS) software (Version 17). Statistical test employed for the comparison between clinical and control group was independent sample t-test. The mean difference between the variables was considered statistically significant a p<0.05.
3. Results
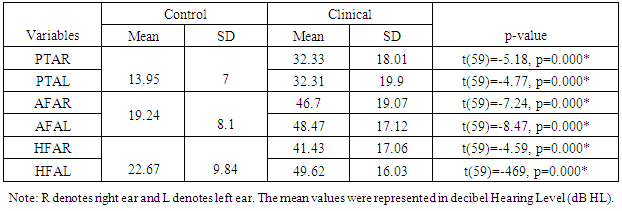
- The puretone thresholds [Puretone Average-PTA of three frequencies viz., 500 Hertz (Hz), 1 kilo Hertz (kHz) and 2 kHz; All Frequency Average-AFA of frequencies 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, 6 kHz, 8 kHz, 10 kHz, 12.5 kHz, 16 kHz and 20 kHz and High Frequency Average-HFA, i.e., puretone average of high frequencies such as 4 kHz, 6 kHz, 8 kHz, 10 kHz, 12.5 kHz, 16 kHz and 20 kHz], speech audiometric results [Speech Recognition Thresholds-SRT, Speech Discrimination Score- SDS], Signal to Noise Ratio (SNR) of DPOAE and latencies of ABR (absolute and interpeak) were compared between right and left ears in the control group using independent samples t-test at a significance level of p<0.05. The mean difference between the right and left ears was found to be statistically not significant in all indices. Hence a combined mean value of the mentioned tests for control group was calculated for the purpose of comparison with the clinical group.
3.1. Puretone Audiometry
- The comparison of PTA, AFA and HFA between the clinical and control group was done using independent student t-test and was found to be statistically significant (Table 1). The clinical group had poor scores on all three indices.
|
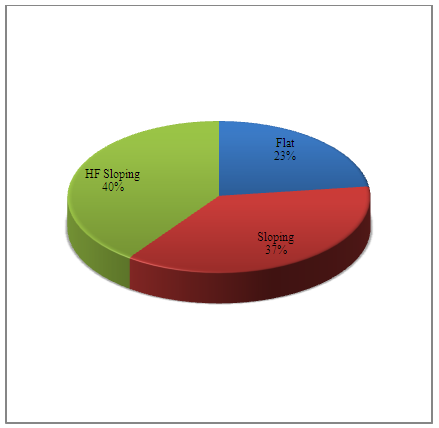 | Figure 1. Audiogram configuration in the clinical group |
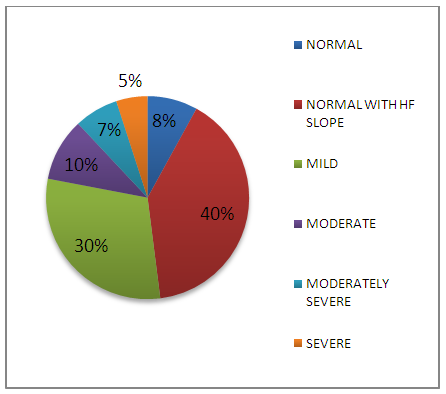 | Figure 2. Hearing sensitivity in clinical population |
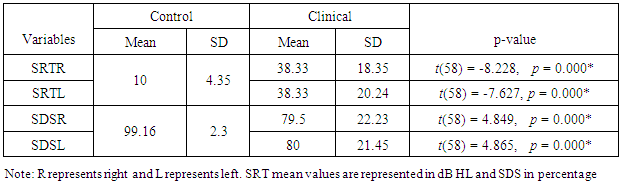
3.2. Speech Audiometry
- Mean values of SRT and SDS of control group were compared with the clinical group using student’s independent t-test and found to be statistically significant (p<0.05). SRT in decibel Hearing Level (dBHL) and SDS in percentage were poorer in the clinical group (Table 2).
|
3.3. Tympanometry
- The tympanogram type found in all control and clinical group subjects were ‘A’ type except that four ears of the clinical population had ‘B’ type.
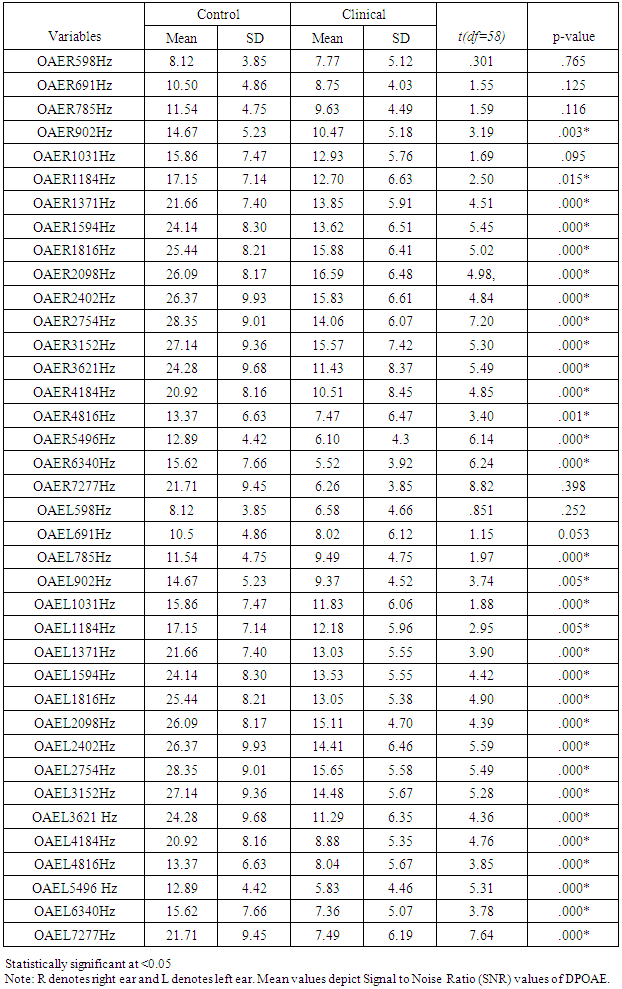
3.4. Distortion Product Otoacoustic Emissions (DPOAE)
- Comparison between the control and clinical group were done using independent t-test and found that the results were statistically significant at majority of test frequencies (Table 3). The clinical group had poor scores.
|
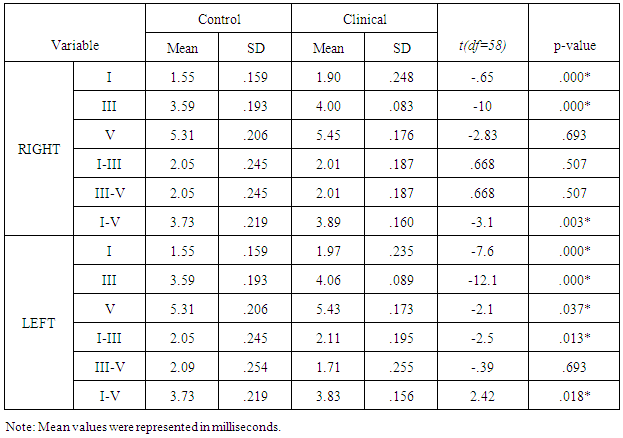
3.5. Brainstem Evoked Response Audiometry (BERA)
- Independent t-test was employed to compare the mean latencies between the clinical and the control group and significant differences were found. The absolute latencies of waves I & III in right ear were found to be significantly different. Similarly, absolute latencies of peaks I, III and V were significantly different in left ear. The interpeak latencies of III-V & I-V were prolonged in right and I-III and I-V were prolonged in the left ear. The clinical group had poor values for all indices (Table 4).
|
3.5.1. Morphology and Replicability of BERA Waveform
- Morphology and replicability of the BERA waveforms were rated as good, fair and poor based on the subjective evaluation of the examiner. The control group had good morphology and replicability whereas the clinical group showed all three ratings such as good, fair and poor. Fifty three percent of the clinical population had good morphology, 20% had fair and 27% had poor morphology. The replicability was found to be good in 100% of the clinical population.
4. Discussion
- Review of literature indicated that smokers have 15.1% greater chance of hearing impairment than non-smokers [13]. In the present study, all three indices of puretone audiometry viz., PTA, AFA and HFA were significantly poorer than the control group indicating pathological influence of smoking across all frequency regions. Similar findings have been reported by Oliveira and Lima [14] and Fabry et al [15]. In the present study, among subjects with hearing loss, majority had SNHL (87%) and only 13% had mixed hearing loss. A study done by Kumar et al [16] on 108 smokers revealed similar findings. In their study, 77.5% of smokers had SNHL and only 18.3% had mixed hearing loss. The conductive pathology observed in minority of subjects could be due to histopathological changes in the mucosal lining of the middle ear [4]. The sensorineural component observed in majority of subjects could be attributed to the factor that nicotine and carbon monoxide that result from smoking can restrict the blood flow to the inner ear, thereby damaging hair cells in the cochlea. The free radicals from smoke can also damage hair cells. The function of auditory nerve can get compromised due to pathological influence on neurotransmitters [17]. The degree of hearing impairment was not very drastic as 8% of smokers had normal hearing sensitivity across the frequency range of 250-8000 Hz; 40% had normal hearing sensitivity between 250-4 kHz and loss confined only to 6 kHz and 8 kHz and 30% had mild hearing impairment. Seventy eight percent of subjects had either normal, only high frequency loss or mild hearing impairment thus revealing comparatively milder effect of smoking on hearing thresholds. Similar findings have been reported by Kumar et al [16]. In their study, mild form (26-40 dB loss) was the most common (56.5%), while the severe type was the least common (2.8%) in the smokers. In the present study, high frequency hearing impairment was more pronounced as 77% of the smokers had sloping audiogram configuration. Similar findings have been reported by Nakanishi et al [3] and it was dose-dependent. Similarly, Fransen et al [2] conducted a study in 4083 subjects between 53 and 67 years and reported that smoking significantly increased high-frequency hearing loss with dose-dependent effect. In the present study also, along with ultra-high frequencies (10-20 kHz), involvement of 6 kHz and 8 kHz were clearly evident based on audiogram configuration. Cigarette smoking has been shown to affect high frequencies in the 0.25-8 kHz range [18], [3], [1]. There is evidence that the cochlear artery that terminates in the high frequency region of the cochlea is very susceptible to the effects of atherosclerotic changes, which are seen in smokers [19]. This could probably explain the pathophysiological mechanism for greater high frequency involvement. Longitudinal study on smokers will be helpful in ascertaining the pathophysiological progression and involvement of low and mid frequencies.In the present study, SRT and SDS were measured, analyzed and compared between the non-smokers and the smokers. Similar to findings obtained from the puretone audiometry, the clinical group obtained poor scores in SRT and SDS. Presence of hearing loss may be the primary reason for poor speech audiometric results. In this study, individuals who have the habit of smoking had hearing loss at speech frequency regions of 500 Hz, 1000 Hz & 2000 Hz and this would have influenced the results. Further, since the high frequencies were majorly affected, consonant identification will definitely be difficult. In the present study, damage to the cochlear outer hair cells is clearly evident as DPOAE (SNR) of the smokers was significantly poorer when compared to the non-smoking population. Nicotine-induced vasospasm, atherosclerotic narrowing, and/or thrombotic occlusion of blood vessels may reduce blood supply to the cochlea in smokers [19], [20], [18], [21], [3]. Cigarette smoking has also been shown to increase carbon monoxide in the blood supply [20] and to increase blood viscosity [22], [7], [23]. Such changes could lead to hypoxic damage to the cochlea [20] and can influence DPOAEs [24], [25]. Thus, reduced DPOAE amplitudes in smokers may be attributable to chronic hypoxic insult to the cochlea associated with vascular insufficiency.In the present study, SNR values were reduced more in the high frequency region than the low frequencies. Negley et al [8] have shown that loss or reduction of blood supply to the cochlea may be the primary mechanism of hearing damage in smokers. Twenty-four healthy adults, 12 smokers and 12 non-smokers within the age range of 20-30 years were considered. DPOAE results showed small, but significant, decline in DPOAE levels in smokers as compared to non-smokers. I/O detection thresholds were also significantly elevated at high frequencies in smokers as compared to their non-smoking counterparts. The greater involvement of high frequency regions could probably be attributed to the explanation provided by Zelman [19] that the cochlear artery that terminates in the high frequency region of the cochlea is very susceptible to the effects of atherosclerotic changes, which are seen in smokers. Irrespective of the hearing loss, the ABR was administered in all the subjects bilaterally. Absolute latencies of peaks I and III as well as I-V interpeak latencies were prolonged bilaterally. These findings probably indicate dysfunction at the level of auditory nerve and brainstem, thus revealing retrocochlear auditory involvement in the smoking population. Howard et al [21] showed that smokers were most susceptible to atherosclerotic damage and that as the numbers of pack years of smoking increased, atherosclerotic damage also increased, so that oxygen deprivation may affect both the cochlear hair cells and the spiral ganglion cells. According to Negley et al [8], nicotine and carbon monoxide (CO) in the cigarette depletes the oxygen level in the cochlea (hypoxia) which affects the ponto-medullary portion of the brain by slowing the information processing skills there by prolonging the BERA latencies.
5. Conclusions
- In the present study, pathological auditory involvement is clearly evident in smokers. The puretone audiometric findings indicated reduced hearing sensitivity. This was particularly evident at high frequency regions as demonstrated by audiogram pattern (majority had sloping hearing loss). Sensorineural hearing loss was more prevalent in smokers. Speech audiometry also demonstrated pathological effect. DPOAE findings indicated involvement of cochlear outer hair cells. ABR indicated probable involvement of auditory nerve and brainstem. Hence an audiological evaluation comprising of immittance audiometry, DPOAE, puretone and speech audiometry with BERA could comprehensively assess auditory deficits in smokers. Periodic evaluation in them could demonstrate presence/progress of auditory deficits. Such information may prove to be another effective factor in helping smokers to quit the habit. Thus audiological evaluation could serve a great social cause. Future endeavours should focus on longitudinal studies assessing smokers in greater number and detail, thus unraveling minute auditory pathophysiological aspects along with progressive deterioration. Involvement of central auditory structures could be assessed in greater depth. Confounding effect of passive smoking, particularly in control group subjects, needs to be scientifically assessed for avoiding its influence on results.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML