-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2015; 4(1): 1-6
doi:10.5923/j.otolaryn.20150401.01
Tracheostomy Decannulation: When and How?
Shreeharsha Maruvala, Ravishankar Chandrashekhar, Ruchi Rajput
Department of Otorhinolaryngology, Bangalore Medical College and Research Institute, Bangalore, Karnataka, India
Correspondence to: Ravishankar Chandrashekhar, Department of Otorhinolaryngology, Bangalore Medical College and Research Institute, Bangalore, Karnataka, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Although tracheostomy is considered to be the most common surgical procedure performed on critically ill patients, there is no general consensus as to when a tracheostomy tube can be safely removed. It is reported that approximately 10% of mechanically ventilated critically ill patients need tracheostomy for prolonged airway and ventilatory support. Prolonged tracheostomy tube placement may lead to increased risk of late complications, including tracheal stenoses, bleeding, fistulas, infections, accidental dislodgement, and mechanical problems with cuff, aspiration and pulmonary complications. In general, majority of patients with tracheostomy who are discharged from Intensive care units (ICU) can be successively decannulated. Literature shows a considerable diversity in criteria for decannulation. It is a multifactorial process and the protocols may vary from one setting to another. Respiratory therapists and physicians and Otolaryngologists are the group of clinicians most directly involved in decannulation process. Considerable differences in their decannulation practices are observed which necessitates the need for the development tracheostomy decannulation guidelines which can be applied in different clinical settings in which tracheostomy was performed. This paper aims to review this process and evolve a method which can be applied in different clinical settings.
Keywords: Tracheostomy, Decannulation, Respiratory obstruction, Prolonged mechanical ventilation, Spinal cord Injury
Cite this paper: Shreeharsha Maruvala, Ravishankar Chandrashekhar, Ruchi Rajput, Tracheostomy Decannulation: When and How?, Research in Otolaryngology, Vol. 4 No. 1, 2015, pp. 1-6. doi: 10.5923/j.otolaryn.20150401.01.
Article Outline
1. Introduction
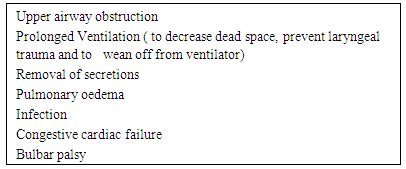
- Tracheostomy was performed first in ancient Egypt and is one of the oldest surgical procedures [1]. It is described in many ancient surgical texts [2]. At the beginning of last century the principles of the operation were described by Chevalier Jackson [3] and it is practiced till date. With the growth of new techniques like percutaneous tracheostomy and improved ventilatory care, this procedure is performed more frequently in recent times for wider indications. This has resulted in more health care professionals being exposed to challenges in tracheostomy care and Decannulation procedures. Literature shows a considerable diversity in criteria for decannulation. It is a multifactorial process and the protocols may vary from one setting to another. Respiratory therapists, physicians and Otolaryngologists are the group of clinicians most directly involved in decannulation process. Considerable differences in their decannulation practices are observed which necessitates the need for the development of tracheostomy decannulation guidelines which can be applied in different clinical settings in which tracheostomy was performed [4-8]. This paper aims to review this process and evolve a method which can be applied in different clinical settings.The indications for placement of a tracheostomy tube include; to bypass an upper-airway obstruction, failure to wean from mechanical ventilation and impaired neurologic status, an inability to handle excessive secretions.
|
2. Decannulation Protocol
- The protocol should include several evaluations:1. Baseline oxygen saturation level (SaO2)SaO2 must be over 92% breathing room air or with oxygen supplementation in patients with previous lung disorders, such as chronic obstructive pulmonary disease (COPD), in order to assure an appropriate tissue oxygenation.2. Need for mechanical aspirationIt can be assessed as number of tracheal aspirations over 24 hours; a cut-off is not established. Abundant bronchorrhea, and need for frequent aspiration are considered a relative contraindication to decannulation.3. Assessment of protective reflexesThis means in particular to evaluate the effectiveness of the cough reflex, by assessing the intensity of the cough either spontaneous or induced by tracheal aspiration. The absence of an effective cough is a contraindication to decannulation. A PCF over 160 L/min, eventually with adjuvant techniques such as manually or mechanically assisted cough, is in favour for decannulation.4. Chest X-ray (when indicated)The presence of abnormalities at the chest x-ray, such as pneumonia or pleural effusion, may contraindicate decannulation.5. Flexible endocopy (when indicated)Essential to evaluate vocal cord mobility and tracheal patency. Vocal cord paralysis in adduction does not allow the patient to be decannulated.6. For a successful decannulation process, swallowing evaluation must be combined with a pathophysiological study of respiratory function.It is recommended to proceed with decannulation in subjects with a suitable level of consciousness, after clinical assessment of tolerance to the progressively longer capping of the cannula (up to at least 48 consecutive hours).A system for patient assessment prior to decannulation is outlined in Figure 1.
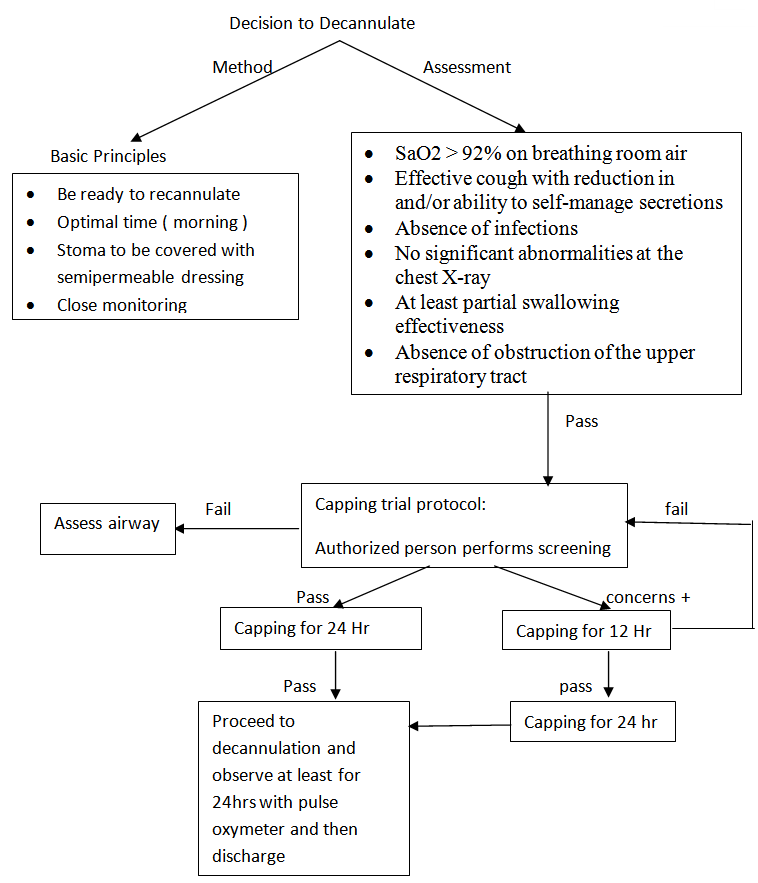 | Figure 1. Decision making in the process of decannulation |
3. Decannulating in Patients in Whom Tracheostomy was Performed to Bypass Upper Airway Obstruction
- Before considering capping of tracheostomy, it is important to establish that the upper airway (i.e., glottis, vocal cords, and subglottic space) is patent. The presence of a tracheostomy tube can cause complications that may result in upper airway obstruction. [9] The upper airway can be checked noninvasively by fully deflating the cuff on the tracheostomy tube and placing a gloved finger over the tracheostomy tube opening to deflect air through the upper airway and vocal cords, allowing phonation. [10] This technique helps identify patients who can tolerate occlusion of the tracheostomy tube and also those who may benefit from having a tracheostomy tube with a smaller external diameter. [11] If the patient is unable to phonate, has stridor or laboured breathing, or manifests any respiratory distress, a thorough endoscopic examination of the airway, including the vocal cords and subglottic space, is recommended. [9] If the airway patency is compromised by stenosis, granulation tissue, or abnormal vocal cord movement, otolaryngologist should consider the different options available for further evaluation and treatment. In one study, 67% of patients with tracheostomies were found to have airway abnormalities during airway endoscopy. Findings included tracheal granulomas, tracheomalacia, tracheal stenosis, and vocal cord dysfunction [14]. Some of the abnormalities visualized, such as minor mucosal trauma from the tracheostomy tube or suction catheter, may not be clinically important and usually do not prevent decannulation. It has been demonstrated that patients who successfully pass a tracheostomy-tube- occlusion protocol can be safely decannulated without first undergoing fiberoptic endoscopy. The procedure is safe, requires only topical anaesthesia. If no pathology is found on endoscopy, the tube may be downsized and cuff is fully deflated to enhance air flow around the occluded tube. Till they are ready for decannulation, in spontaneously breathing patients who tolerate occlusion of the tracheostomy tube as described above, a one-way speaking valve may be placed onto the tracheostomy tube with a fully deflated cuff. This allows for air flow into the tube during inspiration; however, air flow now exits through the upper airway and vocal cords upon exhalation, producing speech.
4. Process of Weaning and Decannulation in Long Term Ventilatory Support
- The placement of a tracheostomy tube facilitates the transfer of the patient from the intensive care unit to a weaning facility such as a step-down unit or a long-term care hospital [16]. Here, a multidisciplinary team manages medical care, rehabilitation, and weaning the patient from prolonged mechanical ventilation. Chronic comorbidities and the lack of evidence-based weaning and decannulation guidelines make it difficult to predict weaning outcomes of individual patients [17-19]. Clinically stable patients undergoing prolonged mechanical ventilation usually begin the weaning process by spending increasing amounts of time on a spontaneous breathing trial via humidified tracheostomy mask. The term ‘weaning’ can mean; i)a reduction in support from mechanical ventilation (or CPAP/assisted spontaneous breathing modes), ii) a generic term for the period of time as the patient progresses towards decannulation, iii) a reduction in the size of the tracheostomy tubes. The latter term is referred to as ‘down-sizing’ in this review. Evaluating the ‘need’ for a tracheostomy and planning weaning should be part of the daily assessment. Some patients may tolerate rapid decannulation, especially if their ventilation period has been short or if they do not suffer significant lung or airway pathology or neuromuscular problems. Others, particularly those with underlying cardiopulmonary disease, muscle weakness, neurological deficits, upper airway oedema or problems managing airway secretions, will take much longer to wean and it is important that the process is both planned and sequential. The procedure is usually straightforward, but adequate assessment and preparation as outlined below is required to maximise success.
|
5. Patients with Neuromuscular Insufficiency
- Airway clearance: Extreme caution is required when making the decision to remove a Tracheostomy Tube (TT) from Spinal Cord Injury (SCI) patients who are clinically aspirating, and a team approach is recommended. Airway clearance issues must be considered and a closely monitored decannulation process instituted. A strong cough may be difficult to achieve owing to varying degrees of abdominal muscle strength. A technique called ‘assisted coughing’ is used to help clear secretions for those with abdominal weakness. This involves providing a firm upward thrust below the diaphragm at the precise moment of coughing. Additional pressure may also be exerted over the chest wall to increase the generation of intrathoracic pressure [23]. It is vital that the patient learn to perform this technique effectively to avoid sputum retention. A normal cough requires a precough inspiration or insufflation to about 85 to 90% of total lung capacity. Glottic closure follows for about 0.2 second and sufficient intrathoracic pressures are generated to obtain “peak transient expiratory flows” or PCFs upon glottic opening that are normally 360 to 1000 L/min.'' PCFs are reduced by the inability to adequately inflate the lungs (reduced vital capacity), abdominal (expiratory) muscle weakness, and often the inability to adequately adduct the vocal cords and close the glottis to retain a deep breath before generating the cough. In addition, bronchospasm or any conditions that result in irreversible upper or lower airway obstruction also reduce PCF. It has been shown that for patients with paralytic conditions, PCF can be significantly increased by encouraging maximal inspirations and flows can be further increased by appropriately timing an abdominal thrust to glottic opening (manually assisted coughing)1. Vital capacity can be improved by providing incentive spirometry, which increases respiratory muscle power.
6. Discussion
- Clinicians indicated in one survey that, in determining whether to decannulate a tracheostomized patient, the patient's level of consciousness, ability to tolerate tracheostomy capping, cough effectiveness, secretions, and oxygenation needed to be evaluated. Although the ability to tolerate tracheostomy capping was judged to be an important determinant of tracheostomy decannulation, it did not influence clinicians' recommendations in all clinical scenarios. Previous studies and guidelines have also suggested that maximal expiratory pressure, peak cough flows, arterial blood gases, and upper airway endoscopy may be useful in the decannulation decision-making process, in spite of these factors requiring special equipment and expertise and are more complicated than the simple bedside criteria [14]. These findings highlight the need for clinical studies in tracheostomy care to guide clinical decision-making.Therapist-driven weaning protocols, such as those involving spontaneous breathing trials or decreasing levels of pressure support, have been implemented in the post-acute-care setting and have been shown to shorten the time taken to wean patients from prolonged mechanical ventilation [21, 22]. Not all patients are suitable for a weaning protocol, and some need an individualized approach given the complexity of the patient population. The presence of respiratory muscle weakness, slow recovery from chronic critical illness with multi-organ dysfunction, anxiety from prolonged ventilator dependence, chronic anemia, or cardiac dysfunction may necessitate a more gradual weaning strategy. The principle involved is to gradually decrease the amount of pressure-support ventilation prior to withdrawal of full ventilator support. All patients undergoing weaning from mechanical ventilation should be carefully monitored using continuous pulse oximetry. In spinal injuries, inability to manage saliva and dysphagia are to be considered before the implementation of the decannulation, although they do not represent an absolute contraindication to decannulation. Another retrospective study shows how a multidisciplinary approach with a swallowing assessment can lead to a high decannulation percentage (99.5%), even more quickly (48 days after insertion) compared to an approach without a multidisciplinary protocol (88% with 94 days interval from insertion to decannulation) [24]. However, there is currently insufficient evidence for direct correlation of decannulation with dysphagia or aspiration.
7. Conclusions
- It is extremely difficult to device a prospective randomised controlled trial to study the various factors which influence the process of decannulation and compare them with controls. However, carefully devising protocols in two situations, spinal cord injury and respiratory insufficiency and evaluating them will be helpful in managing this situation more effectively. One more issue needs to be addressed is the management of problems associated with long term tracheostomy tube placement (tracheal stenosis, granulations, bleeding, fistulas and tracheomalacia). The protocols should incorporate the present preventive methods or practices used to avoid such complications and also test their efficacy. The removal of the tracheostomy cannula is an important rehabilitation goal, but cannot always be performed. However, the severity of the clinical and neurological state seems to have a significant influence on decannulation failure. Decannulation is also possible in selected cases of patients in a vegetative or in a minimally conscious state after verifying a reasonable effectiveness of cough and spontaneous swallowing. In any case, rehabilitation of patients with a tracheostomy cannula requires a close integration among the various professional figures with a particular regard to the assessment of the dysphagia. As a matter of fact, decannulation is a complex and multidisciplinary process, which considers various aspects from cognitive to critical issues such as protecting the respiratory tracts. Swallowing represents a fundamental aspect in this process. There are currently few documents that indicate shared protocols for the assessment of swallowing in the decannulation process. We propose that a patient's ability to tolerate capping, level of consciousness, cough effectiveness to manage secretions, and swallowing be tested in a clinical trial as four simple bedside factors to consider in determining whether to decannulate a tracheostomized patient.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML