-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2014; 3(5): 73-76
doi:10.5923/j.otolaryn.20140305.03
The Maintenance of Nutrition in Patients with Head and Neck Malignancy – A Review
C. Ravishankar, N. Dhanapala, V. Priyadarshini
Department of ENT, Bangalore Medical College & Research Institute, Bangalore, India
Correspondence to: N. Dhanapala, Department of ENT, Bangalore Medical College & Research Institute, Bangalore, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Malnutrition is common in patients with head-and neck cancer because of pre-existing nutritional deficiencies, tumour location, and treatment-related side effects. When administration of intensive multimodality treatments is concurrent, severe and often debilitating effects can compromise the patient’s ability to maintain adequate nutrition and hydration. Thus, nutritional support is an integral component of care in these patients. Aggressive enteral feeding regimens are required to address malnutrition and nutritional decline, but there is disagreement regarding the best route (oral or tube feeding), when to initiate aggressive feeding (proactive or reactive), and the type of tube placement. This work aimed at reviewing the causes of malnutrition among patients with head and neck malignancy, impact of malnutrition and analysing the benefits and risks of enterinc feeding support for adult patients with squamous cell carcinoma of the head and neck who receive surgical or combined chemotherapy and radiotherapy with curative intent.
Keywords: Nutrition, Head and Neck Malignancy, Malnutrition
Cite this paper: C. Ravishankar, N. Dhanapala, V. Priyadarshini, The Maintenance of Nutrition in Patients with Head and Neck Malignancy – A Review, Research in Otolaryngology, Vol. 3 No. 5, 2014, pp. 73-76. doi: 10.5923/j.otolaryn.20140305.03.
Article Outline
1. Introduction
- Weight loss in adult patients, weight loss or failure to gain weight normally in children is a frequent adverse effect of cancer [1]. Most patients of head and neck malignancy are malnourished at diagnosis. These malignancies are notorious for dysphagia due to mechanical obstruction, sensory impairment, odynophagia and/or trismus. The co-existent alcohol abuse and long term tobacco abuse in this population further worsens the nutritional status [2]. Weight loss during treatment for HNSCC is a major concern; substantial weight loss in 75-80% of patients has been widely reported. While these obstacles are often due to the cancer itself, the common treatments for HNSCC, including surgery, radiotherapy (RT), and chemotherapy, also lead to changes that further complicate and challenge oral intake [3]. Despite the fact that there is wide spread awareness regarding the major problem posed by the poor nutritional status of these patients there exists a pervasive attitude that some weight loss is inevitable when protracted treatment is given to the terminally ill. This attitude precludes aggressive intervention and focus towards the nutritional status of the patients.In this review article we have analysed three important factors regarding the nutrition of patients with head and neck cancers. 1) the factors contributing to the malnutrition, 2) the relationship between malnutrition and the outcome of the patient and 3) the methods of delivering enteral nutritional support.
2. Factors Contributing to Malnutrition
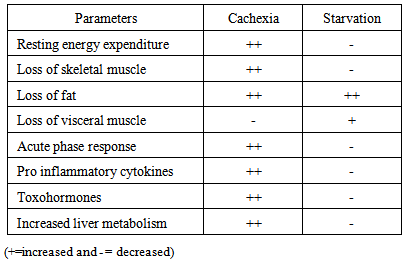
- One possible reason for malnutrition in patients with head and neck cancer is the location of the tumour. Oropharyngeal cancer may cause anorexia, nausea, inadequate mastication, xerostomia, dysgeusia, dysphagia or odynophagia. Diminished oral intake and avoidance of firm solids correlated with malnutrition [4].The pathogenesis of cancer cachexia is not strictly a function of tumour burden. Cancer cachexia is defined as “a state of maladaptation to the fasting state with ongoing mobilization of energy reserves” [4]. In normal subjects, a forced reduction in caloric intake leads to a reduction in caloric expenditure; but in cancer patients, this normal adaptation may be blunted or blocked [1]. Glucose is metabolized in tumor tissue predominantly via anaerobic glycolysis. This predominance of anaerobic metabolism over aerobic metabolism persists even if tumor cells are well oxygenated in tissue culture, and it is explained by concentrations of enzymes which control cellular metabolism. The lactic acid thus produced is metabolised into glucose by the liver and kidney in an energy requiring process called Cori cycle which accounts for a 10% increase in energy expenditure in tumor bearing individuals [5]. Proinflammatory cytokines, secreted by either the tumor or the host, initiate an exuberant systemic inflammatory response, which also results in an increased production of acute-phase proteins in the liver, further depleting essential amino acids [6]. Proinflammatory cytokines such as Tumor Necrosis Factor-a (TNF-a), Interleukin-6 (IL-6), interleukin-1b (IL-1b), and Interferon-g (IFN-g) are implicated because of their ability to cause muscle wasting [7]. Toxohormones are tumor derived factors such as Lipid Mobilising Protein (LMP) and tumor derived Proteolysis Inducing Factor (PIF). These hormones further mediate the lipolysis and proteolysis [6]. Low levels of leptin are seen in weight loss, which stimulates feeding through increased hypothalamic or exigenic signalling. In cancer cachexia, however, leptin levels are inappropriately altered [6].Post Treatment undernutrtion may be due to various factors. Surgery, depending on the tumour site, procedure, and approach, may significantly alter the anatomy and lead to scarring that negatively impacts swallowing [2]. The acute reaction of the aerodigestive tract as a result of undergoing radiotherapy (RT) is associated with diverse gastrointestinal symptoms and decreased food intake, resulting in deterioration of the patient’s nutritional status [8]. Adjuvent or neoadjuvent chemotherapy is being used in conjunction with radiotherapy. Chemotherapy enhances the negative effects of radiation. Additionally, chemotherapy causes nausea and vomiting and reduces the desire to eat.
|
3. Impact of Malnutrition on Outcome of Patient
- Some studies of patients with head and neck cancer have found correlations between malnutrition and increased postoperative morbidity, mortality, length of hospitalization, and decreased survival at two years [9]. Linn [4] prospectively evaluated 79 men who had surgery for head and neck cancer, using his Protein Energy Malnutrition Scale .Malnourished elderly patients had the worst surgical outcomes. Goodwin [9] retrospectively studied 50 consecutive patients with stage III, IV or recurrent squamous cell carcinoma of the head and neck, 47 of whom had a variety of treatments, including induction chemotherapy, surgery, and/or radiation. Treatment-related complications in the patients with severe malnutrition based on the Prognostic Nutritional Index were always major and more frequent, compared to the 36 patients with no or mild malnutrition. Malnutrition can result in poor postoperative results, impaired and delayed wound healing, and a higher incidence of postoperative complications [10].
4. Role of Enteric Nutritional Support
- Nasogastric tube plays only a limited role in enteric nutrition since they can be used only for a short duration. It is contraindicated in those with gastric stasis or obstruction.The more commonly used methods for long term enteral feeds are gastrostomy and jejunostomy. The various techniques of performing the gastrostomy are mainly percutaneous endoscopic gastrostomy, percutaneous fluoroscopic gastrostomy, percutaneous radiologically assisted gastrostomy and open gastrostomy. Of these methods Percutaneous endoscopic gastrostomy (PEG), surgically-inserted (open/laparoscopic) gastrostomy (SIG) and radiologically inserted gastrostomy (RIG) were compared in a study [2] and the incidence of minor complications were found to be same in the three methods but the incidence of major complications and death was found to be 0% in PEG, 10% in SIG and 11% in RIG. In comparison to nasogastric tube PEG there is less interruption from tube displacement compared with nasogastric feeding. Nasogastric tube has a feeding discontinuation rate of 15% and swallowing problems of 17% as compared to 0% discontinuation of PEG. There may also be less reflux and feed aspiration, suggesting that overnight feeding is relatively safer [11]. Percutaneous Endoscopic Gastrostomy was introduced in 1980 by Guaderer and Ponsky. The method of performing a PEG by the “pull through” technique is as 6 step process [12]. 1. Endoscope is introduced into the stomach and stomach inflated with air.2. Using this transillumination as the guide a stab incision of 5-6mm is made in the epigastrium.3. Through this incision a sheathed needle with thread is introduced into the stomach, this thread is grasped by the endoscopist and drawn out through the mouth with the scope.4. The thread is now tied to the fixation loop attached to the tapered dilator end of the PEG tube.5. The distal end of the thread is pulled to position the tube which eventually emerges through the abdominal wall and the bumper stops at the inner gastric wall.6. External fixation device enables a close connection between the wall of the stomach and parieties.The argument against PEG is the anecdotal observation of prolonged dependence on PEG and increased need for pharyngo-esophageal dilations for persistent dysphagia [12]. Another rare observation is the possibility of tumor seeding.PEG tubes are made of silicone or polyurethane. They range from 12F to 28F in diameter. Their position is secured internally on the anterior gastric wall by either a bumper or an inflated balloon and externally on the anterior abdominal wall by a bumper or a bolster. External markings on the tube indicate the length of the transabdominal wall tract [15].In end-stage and terminal disease patients PEG is not feasible due to both pain and bleeding, as there is obstruction at the oropharyngeal and oesophageal region. In a study conducted by Boraz et al [14] PEG was not possible in 86.2% patients because of obstruction. In the literature PEG and PFG (Percutaneous Fluroscopic Gastrostomy) have become gold standard owing to cost analysis, complication rates and operation time. Yet, in cases of obstructing cancers (i.e. head/neck, oesophageal and gastric), obesity or previous laparotomy, PEG and PFG are not feasible and a surgical gastrostomy has to performed [14].
|
5. The Techniques of Jejunostomy
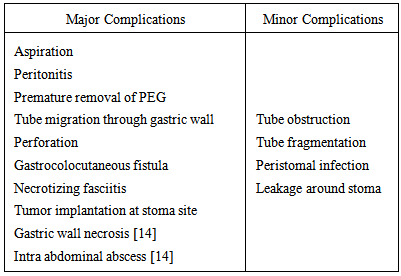
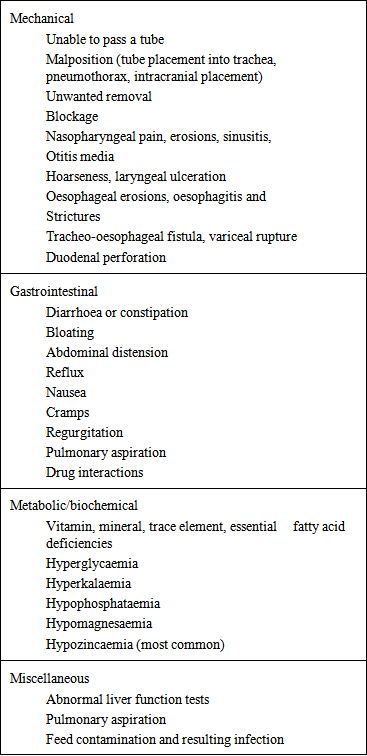
- Percutaneous endoscopic jejunostomy: This is technically more demanding than PEG because of the narrow lumen and motility of jejunum. There are various methods to insert PEJ. The method which is preferred in cases where the surgeon wants to maintain a virgin stomach as a conduit where esophagectomy is a possibility is the Direct Percutaneous Endoscopic Jejunostomy. Another method is the PEG with jejunal extension (PEG-J), although strictly speaking this is not a jejunostomy but instead 6F to 9F tube is inserted through the PEG and advanced into the jejunum. This prevents the risk of aspiration by delivering the food beyond the pyloris [16]. Yet another method is the percutaneous Fluroscopic Jejunostomy.Surgical Jejunostomy can be done either as a open procedure or Needle Catheter Jejunostomy (NCJ). The 6 steps in performing an open jejunostomy by the Whitzel technique are as follows:1. Several centimetres below the ligament of Treitz the jejunal loop is accessed. 2. Purse string suture is applied along the selected site on the anti mesenteric border.3. Small enterostomy is made and tube inserted 15-20cm distally following which the purse string suture is tied.4. Serosal tunnel is created 3-5 cm proximally from the site of catheter exit.5. Catheter is delivered via a stab incision onto the abdominal wall.6. Jejunal loop around the tube exit site is anchored to the parietal peritoneum.Regardless of the method of placement the main indications for jejunostomy are tracheal aspiration, reflux esophagitis, insufficient stomach from previous resections, following major surgeries and unresectable gastric and pancreatic tumors [16].The complications of enteral feeding are listed in the following table (Table 3) [10]:
|
6. Conclusions
- The various options available for enteric nutritional support are as follows:
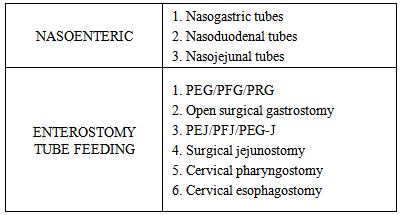 1. Nasogastric tube versus PEG: If 2 or more factors indicate the need for prolonged enteral support then PEG is preferred over nasoenteral feeding. 2. PEG versus Jejunostomy feeding: Jejunostomy can be performed by open surgery or endoscopically. Patients on jejunostomy feed received a significantly higher proportion of their daily goal caloric intake, had a significantly greater increase in serum prealbumin concentration, and a lower rate of pneumonia than patients fed by continuous gastric tube feeding.3. Pre operative nutritional supplementation: Caution must be exercised when preoperative nutritional support is required in cases of hypopharyngeal tumors or patients requiring laryngopharyngectomy. This is a tricky situation, where a preoperative PEG will be a hindrance if a gastric pull-up is necessary. Similar caution should be advocated when performing a PEJ for these cases if there is a possibility of a free jejunal flap. Feeding through small bore feeding tubes or via the nasoenteric route is preferred in this scenario.4. Role of open gastrostomy: Despite the lower complication rate and higher success rate of the percutaneous techniques the open surgical procedure still has its own place in the enteric nutrition assistance.The heterogeneity of the patient population, the varied behaviour of the various head and neck malignancies and the plethora of techniques available for enteric nutrition support access necessitates two things, firstly a customised patient specific planning and secondly the need for adequately powered randomized comparative study which compares all the various available options.
1. Nasogastric tube versus PEG: If 2 or more factors indicate the need for prolonged enteral support then PEG is preferred over nasoenteral feeding. 2. PEG versus Jejunostomy feeding: Jejunostomy can be performed by open surgery or endoscopically. Patients on jejunostomy feed received a significantly higher proportion of their daily goal caloric intake, had a significantly greater increase in serum prealbumin concentration, and a lower rate of pneumonia than patients fed by continuous gastric tube feeding.3. Pre operative nutritional supplementation: Caution must be exercised when preoperative nutritional support is required in cases of hypopharyngeal tumors or patients requiring laryngopharyngectomy. This is a tricky situation, where a preoperative PEG will be a hindrance if a gastric pull-up is necessary. Similar caution should be advocated when performing a PEJ for these cases if there is a possibility of a free jejunal flap. Feeding through small bore feeding tubes or via the nasoenteric route is preferred in this scenario.4. Role of open gastrostomy: Despite the lower complication rate and higher success rate of the percutaneous techniques the open surgical procedure still has its own place in the enteric nutrition assistance.The heterogeneity of the patient population, the varied behaviour of the various head and neck malignancies and the plethora of techniques available for enteric nutrition support access necessitates two things, firstly a customised patient specific planning and secondly the need for adequately powered randomized comparative study which compares all the various available options. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML