-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Otolaryngology
p-ISSN: 2326-1307 e-ISSN: 2326-1323
2013; 2(1): 1-5
doi:10.5923/j.otolaryn.20130201.01
Temporal Resolution in Patients with Cerebellopontine Angle Tumors
G. Prem1, N. Shivashankar2, N. Girish3, B. Indira4, S. G. Srikanth5, V. Shanmugham6
1Department of Speech pathology and Audiology, Amrita Institute of Medical Sciences and Research Center, Kochi, India
2Department of Speech pathology and Audiology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India
3Department of Epidemiology, NIMHANS, Bengaluru, India
4Department of Neuro Surgery, NIMHANS, Bengaluru, India
5Senior consultant radiologist, Kanva diagnostic private limited, Bengaluru, India
6Department of Biostatistics, NIMHANS, Bengaluru, India
Correspondence to: G. Prem, Department of Speech pathology and Audiology, Amrita Institute of Medical Sciences and Research Center, Kochi, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
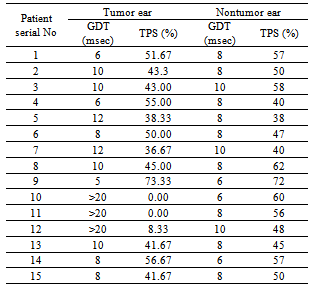
The Gaps-in-Noise (GIN) test is a short and quick assessment tool of temporal resolution. The present study was done to assess temporal resolution skills in patients with brainstem lesions. The study group consisted of patients with radiologically confirmed cerebellopontine angle (CPA) tumors (n=15) demonstrating normal hearing bilaterally. Temporal resolution skills were investigated in these patients using the GIN test. The findings of patient group were compared with results obtained on 100 normal hearing subjects. Gap Detection Threshold (GDT) and Total Percentage Score (TPS) were estimated. The indices in tumor as well as nontumor ears were poor and statistically significant (p<0.001) in comparison to normative data. When tumor ears were compared to nontumor ears, no significant difference was observed with respect to GDT. However, TPS was better in the nontumor ears and statistically significant (p<0.05). The present study demonstrated impaired temporal resolution in tumor and nontumor ears of CPA tumor patients. This indicates the tumor induced vulnerability of auditory system to temporal alterations.
Keywords: Temporal Resolution, Gaps-in-Noise Test, Cerebellopontine Angle Tumors
Cite this paper: G. Prem, N. Shivashankar, N. Girish, B. Indira, S. G. Srikanth, V. Shanmugham, Temporal Resolution in Patients with Cerebellopontine Angle Tumors, Research in Otolaryngology, Vol. 2 No. 1, 2013, pp. 1-5. doi: 10.5923/j.otolaryn.20130201.01.
1. Introduction
- Auditory temporal processing is the perception of the temporal features of a sound (sound envelope fluctuations and starts and stops in ongoing sounds). Temporal resolution, a primary sub-categorization of temporal processing, is the ability of the auditory system to respond to rapid changes in the envelope of a sound stimulus over time[1]. The assessment of temporal resolution may provide insight into the neural integrity of the Central Auditory Nervous System (CANS)[2, 3, 4 & 5] either at cortical or brainstem level.Temporal resolution deficits have been well documented in cortical lesions. In patients with unilateral anterior temporal lobectomy a contralateral ear deficit in temporal resolution has been reported[2]. In animal models, degraded temporal resolution have been reported when both auditory cortices were destroyed[4, 6]. These studies underscore the importance of auditory cortex for fine temporal resolution.Impaired temporal resolution skills in patients with cortical and brainstem lesions have been reported using Gaps-In-Noise (GIN) test[7]. The test is said to be more sensitive to cortical lesions than brainstem lesions. They attributed this to relatively greater extent of cortical lesions and to lesser number of patients tested upon. This study urges the need to further document temporal resolution skills on brainstem lesions. Auditory temporal area being the final destination for the auditory signals, intact transmission along the brainstem auditory pathway is crucial. This implies that brainstem auditory pathway have an important role in intact temporal resolution. In this context, extrinsic brainstem lesions like tumors of the cerebellopontine angle (CPA) can potentially describe the discrete effect of these lesions on temporal resolution. The CPA is one of the most common sites of intracranial tumors and approximately 10% of them originate in this region. The CPA tumors potentially cause direct and/or indirect pathological effects on the auditory nerve and brainstem. In tumors with brainstem involvement, both tumor ear (ipsilateral to the lesion) and nontumor ear (contralateral to the lesion) auditory pathway could get disrupted. This would probably produce auditory deficits either overtly or covertly on both sides. For these reasons, the tumors of the CPA site offer a good clinical milieu to understand the auditory temporal resolution factor. Further, studies linking brainstem lesions and temporal resolution deficits have not been adequately explored and an attempt to understand this aspect is the highlight of the current study.The studies on the effects of hearing impairment on temporal resolution have demonstrated increased Gap Detection Thresholds (GDT)[8, 9, 10 & 11]. To avoid the confounding effects of hearing loss, the present study was designed to evaluate temporal resolution skills in normal hearing CPA tumor patients using GIN test.
2. Methodology
- Ninety eight radiologically (magnetic resonance imaging / computed tomography) confirmed unilateral CPA tumor patients were recruited prospectively for audiological investigations. However, GIN test performance of only 15 patients with normal hearing sensitivity (threshold below 25 dB at octave frequencies from 250 Hertz-Hz to 8 kilo-k Hz in both tumor and nontumor ears) is being discussed in the present article. The patient group comprised of five men and 10 women with age ranging from 15 to 45 years (mean-31.4 years). Three patients had acoustic tumors and 12 had nonacoustic tumors. The data was compared with 100 normal subjects (59 men and 41 women) with age ranging from 15 to 55 years (mean- 33.6 years).Grason Stadler Incorporates (GSI)-61 dual channel clinical audiometer with TDH-50P earphones were used for the study for establishing puretone thresholds as well as for administering the GIN test. The test was carried out in a sound treated two-room situation. The GIN test results were recorded in each ear in both normal hearing group as well as the patient group as per the recommended standard criteria[7]. The stimuli were presented via the compact disc (CD) player connected to the audiometer at 50 dB sensation level (SL) with reference to the puretone average. The indices used were GDT and Total Percentage Score (TPS). Practice list provided in the test was used to train the participants for comprehension of the task. The test comprised of four different lists containing up to 36 signal segments of 6 seconds white noise in each list. The number of gaps of silence in each signal varied from 0-3. The duration of each gap were either 2, 3, 4, 5, 6, 8, 10, 12, 15 or 20 milliseconds (msec) with each silence gap duration occurring six times in each GIN list. Thus each GIN list consisted of a total of 60 gaps and the order of gap durations were randomized. Five seconds gap of silence separated each six seconds noise segment. One list was administered in each ear. While administering the test, the subjects were instructed to listen for any silence gap that may or may not occur within each noise burst. As soon as the gap was detected the subject had to respond by pressing the button. The GDT was calculated by considering: (1) Minimum gap duration correctly identified four out of six times and (2) Similar or better performance for longer gap durations. The TPS was calculated by dividing the total number of gap durations correctly identified by the total number of gap durations presented (n=60) multiplied by 100. False positives were noted down separately. More than two false positives per ear were counted as errors and subtracted from the number of gap durations correctly identified.The results obtained on GIN test for CPA tumor patients (tumor and nontumor ear) were compared with the control group. Similarly, GIN test performance of tumor ears and non tumor ears was compared.The Mann-Whitney test was employed to analyze the age distribution between control and patient groups. The Chi-square test was employed to analyze the gender distribution between control and patient groups. The paired samples t-test was employed to compare GIN scores between right and left ears in control group and between the tumor and nontumor ears in patient group. Independent samples t-test was employed to compare GIN scores between control and patient groups. The mean difference between variables was considered statistically significant at p<0.05.
3. Results
- The Mann-Whitney test showed no statistical difference in age distribution between control and patient groups (U=676, Z=0.615, p=0.538). The Chi-square test revealed no statistical difference in gender distribution between control and patient groups (chi square=3.482, p=0.062). The GIN scores between the right and left ears in the normal group were found to be statistically not significant using paired samples t-test (p>0.05). Thus, the mean normal GDT was estimated to be 5.86 msec (Standard Deviation-SD=1 msec) and TPS 62.49% (SD=6.84%). A 2 SD limit was defined as abnormal and accordingly, a GDT≥8 msec and TPS of 48% and below was used as cut-offs for defining abnormality.
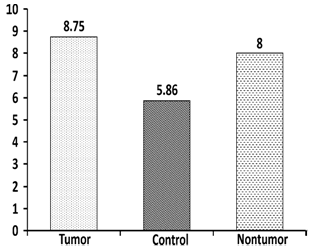 | Figure 1. Mean Gap Detection Threshold (GDT) of Tumor, Nontumor and Control group (msec) |
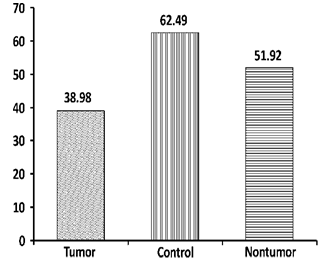 | Figure 2. Mean Total Percentage Score (TPS) of Tumor, Nontumor and Control group (Percentage) |
|
4. Discussion
- The GIN test is a short, quick and robust clinical tool for the assessment of temporal resolution. Because of the use of broadband stimuli for the test, the likelihood of age effects on temporal resolution skills is very low[7]. The upper limit of the GDT in the current study was 8 msec by considering 2 SD. This too corresponds to the limit set by Musiek et al[7]. In the patient group, only three (20%) were acoustic tumors and the remaining 12 (80%) were of nonacoustic type. This probably indicates the preponderance of nonacoustic tumors to present with normal hearing sensitivity.The temporal resolution abilities depend on intact transmission of the signal along the neural pathway. In the current study, although all subjects in the patient group had normal hearing sensitivity their temporal resolution skills, both GDT and TPS, were affected compared to the control group (p<0.001). On inspecting the data of the individual patients on the tumor side, 12 (80%) patients demonstrated impaired GDT. Abnormal TPS was observed in 10 (66.67%) patients. On the nontumor side, 12 (80%) patients had abnormal GDT and six (40%) patients had abnormal TPS. None of the patients demonstrated >20 msec GDT on the nontumor side where as three patients had GDT >20 msec on the tumor side. It appears that irrespective of the tumor side the temporal resolution gets affected bilaterally. Schuknecht and Woellner[12] have observed that when the organ of Corti is intact, 75% of the auditory nerve fibres need to be dysfunctional to produce puretone hearing loss. Thus, in the current study it can be inferred that although auditory nerve fibres were not severely compromised to produce puretone hearing loss, the insult to the auditory structures by the CPA tumor may be responsible for the observed temporal resolution deficits. Similarly, the structural changes resulting from compression of the brainstem by the tumor could compromise the contralateral auditory pathway that could result in GIN impairment in the nontumor ear.Significance of brainstem level auditory structures in influencing temporal resolution have been reported by Walton et al.[5]. They reported that the neural code necessary for behavioural gap detection can be found in the temporal discharge patterns of the majority of inferior colliculus (IC) neurons in the young Cytometric Bead Array (CBA) mouse. Frisina[13], while reviewing encoding of temporal features of the sound in the auditory nerve, cochlear nucleus, superior olivary complex and IC noted that coding for gaps changes from a decrease in spike firing rate for neurons of the peripheral auditory system that have sustained response patterns, to an increase in firing rate for more central neurons with transient responses. These studies demonstrate the significance of brainstem level auditory structures in influencing temporal resolution which lend support to the findings of the current study.When tumor ears were compared to nontumor ears, no significant difference was observed with respect to GDT scores. This implicates the potential influence of the lesion on the nontumor side temporal resolution abilities. However, TPS was significantly better in the nontumor ears. This result should be interpreted with caution. This could probably be attributed to the fact that the TPS in three of the tumor ears, where GDT was beyond 20 msec, were 0%, 0% and 8.33% in subjects 10,11,12 respectively (Ref. Table 1). In them, corresponding nontumor ear TPS were 60%, 56% and 48% respectively. This needs further examination on a larger group of patients. Another intriguing aspect was the finding that GDT and TPS were normal on the tumor side and abnormal on the nontumor side in patient 4 (Ref. Table 1). The radiological and histopathological findings for this patient demonstrated a large (greater than 60 cc) epidermoid tumor in the CPA which engulfed the brainstem. There was no internal auditory meatus involvement on the tumor side. The speech discrimination scores in presence of noise also revealed 90% scores on the tumor side in comparison with 75% scores on the nontumor side. Probably in this patient due to the creepy nature of the tumor, the pathological effect due to brainstem compression was more pronounced on the nontumor side.Further, patient 9 (Ref. Table 1) demonstrated normal GIN test findings bilaterally. The radiological andhistopathological findings demonstrated a small (less than 30 cc) epidermoid tumor in the CPA. Notably, other audiological tests such as puretone and speech audiometry, dichotic digits test and auditory brainstem response administered on this patient were normal bilaterally. This finding poses a question over the sensitivity of GIN test in identifying small nonacoustic tumors. This aspect needs to be validated further by administering the test on larger similar patient group. Musiek et al.[7] reported reduced temporal resolution skills in their study population consisting of 18 patients (nine with cortical and nine with brainstem lesions). All brainstem lesion patients had involvement of auditory brainstem structures caudal to the medial geniculate body. Their overall results showed GIN test to be more sensitive to cortical lesion as opposed to brainstem lesion. Apart from relatively greater extent of cortical lesions and less number of patients tested, this difference could also be due to greater lesion heterogeneity in the brainstem group. Comparatively the current study had a more homogenous patient group and the tumor was confined to the CPA site. Notwithstanding the limitation that the cortical and peripheral lesions have not been evaluated and compared, the present study characterizes the significance of GIN test to differentiate between control subjects and CPA tumors.
5. Conclusions
- The efficiency of GIN test in identifying cortical lesions has been already established. The current study demonstrated impaired temporal resolution in patients with CPA tumors and also showed the vulnerability of the auditory structures to temporal alterations induced by tumor in both tumor and nontumor ears. Since the study included only patients with normal hearing, the confounding effect of hearing loss, if any, has not affected the test results. Despite the small sample size due to its strict inclusion and exclusion criteria, the current study unravelled temporal resolution deficits associated with CPA tumors using the GIN test. Future research should include effect of CPA tumor type, size and extent on GIN test findings. Larger sample size with and without hearing loss would enhance and improve decision making. The GIN test is very easy to administer and could be completed in a short time. The study has brought out the utility of the GIN test as an adjunct clinical tool in identifying temporal resolution deficits associated with brainstem lesions. Temporal resolution is important for speech perception and it is recommended that GIN test be administered as part of the neuroaudiological test battery in evaluation of CANS lesion (cortical or brainstem).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML