| [1] | Beidoe, G., Mousa, S.A. (2012). Current primary open-angle glaucoma treatments and future directions. Clinical Ophthalmology, 6(1), 1699–07. |
| [2] | Tham, Y.-C., Li, X., Wong, T. Y., Quigley, H. A., Aung, T., & Cheng, C.-Y. (2014). Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Journal of Ophthalmology, 121(11), 2081–90. |
| [3] | Koomans, I. (2023, March12). What is glaucoma? World Glaucoma Week (March 12-18, 2023). World Glaucoma Association (WGA), Executive Office. Retrieved on June 13, 2023 from https://www.worldglaucomaweek.org/what-is-glaucoma/. |
| [4] | Miah, M.R., Hasan, M.M., Parisha, J.T. & Chowdhury, S.H. (2022). Socioeconomic Impact of the Coronavirus Pandemic with Multiple Factors on Global Healthcare Policy. Journal of Politics and Law, 15(4), 255-256. doi: 10.5539/jpl.v15n4p242, url: https://doi.org/10.5539/jpl.v15n4p242. |
| [5] | Dipiro, J., Talbert, R.L., Yee, G.C.., Wells, B.G., Posey, L.M., (eds). (2020) Pharmacotherapy: A Pathophysiologic Approach. Glaucoma, 12th ed., McGraw Hill Medical, New York, pp.123-45; Available from; htt://accesspharmacy.mhmedical.com/book.aspx?bookID=2577. |
| [6] | Prum, B.E., Lim, M.C., Mansbberger, S.L., Stein, J.D., Moroi, S.E., Gedde, S. J. (2015). Primary Open Angle Glaucoma Suspect, Preferred Practice pattern. American Academy of Ophthalmology, 123(1), 112-51. |
| [7] | Weinreb, R. N., Khaw, P .T. (2004). Primary Open Angle Glaucoma. The Lancet, 363(22), 1711-20. |
| [8] | Adkins JC & Balfour JA. (1998). Brimonidine. A review of its pharmacological properties and clinical potential in the management of open angle glaucoma and ocular hypertension. Drugs & Aging., 12(3): 225–41. |
| [9] | AGIS Investigators. (2000). The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol., 130:429–40. |
| [10] | AGIS Investigators. (2002). The advanced glaucoma intervention study (AGIS) (12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma). Am J Ophthalmol., 134: 499–512. |
| [11] | AGIS Investigators. (2004). The Advanced Glaucoma Intervention Study (AGIS):13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology., 111: 651–4. |
| [12] | Agnifili L, Costagliola C, Figus M, Iezzi G, Piattelli A, Carpineto P, Mastropasqua R, Nardi M, Mastropasqua L. (2012). Histological findings of failed gold micro shunts in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 250(1): 143-9. doi: 10.1007/s00417-011-1778-6. Epub 2011 Aug 23. PMID: 21861086. |
| [13] | Akman, A., Cetinkaya, A., Akova,Y.A., Ertan, A. (2005). Comparison of additional intraocular pressure-lowering effects of Latanoprost vs. Brimonidine in primary open-angle glaucoma patients with intraocular pressure uncontrolled by Timolol-Dorzolamide combination. South Jersey Eye, vol. 19, no.1 pp.145–51. |
| [14] | Alm A, Camras CB, Watson PG. (1997). Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol., 41(Suppl 2): S105–10. |
| [15] | Alm A. & Stjernschantz J. (1995). Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian Latanoprost Study Group. Ophthalmology., 102(12): 1743–52. |
| [16] | Ambresin A, Shaarawy T & Mermoud A. (2002). Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye of the same patient. J Glaucoma., 11:214–20. |
| [17] | Anand N, Atherly C. Deep sclerectomy augmented with mitomycin C. Eye. 2005; 19: 442–50. |
| [18] | Ansari E. & Gandhewar J. (2007). Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma. Eye (Lond), 21(7): 936-40. doi: 10.1038/sj.eye.6702345. Epub 2006 Apr 21. PMID: 16628239. |
| [19] | Arthur S & Cantor LB. (2011). Update on the role of alpha-agonists in glaucoma management. Exp Eye Res., 93(3): 271-83. doi: 10.1016/j.exer.2011.04.002. Epub 2011 Apr 20. PMID: 21524649. |
| [20] | Aung T, Chew PT, Yip CC et al. (2001). A randomised double masked crossover study comparing latanoprost 0.005% with unoprostone 0.12% in patients with primary open angle glaucoma and ocular hypertension. Am J Ophthalmol. 131:636–42. |
| [21] | Azuma I, Masuda K, Kitazawa Y, Yamamura H. (1993). Double masked comparative study of UF-021 and timolol ophthalmic solutions in patients with primary open angle glaucoma or ocular hypertension. Jpn J Ophthalmol. 1993; 37: 514–25. |
| [22] | Babighian S, Rapizzi E, Galan A. (2006). Efficacy and safety of ab interno excimer laser trabeculotomy in primary open angle glaucoma: two years of follow-up. Ophthalmologica. 2006; 220: 285–90. |
| [23] | Bahler CK, Howell KG, Hann CR, et al. (2008). Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol., 145:114–9. |
| [24] | Barneby H, Orengo-Nania S, Flowers BE, et al. (2005). The safety and efficacy of travoprost 0.004%/Timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol., 140: 1–7. |
| [25] | Bathija, R., Gupta, N., Zangwill, L., Weinred, R.N. (1998) .Changing definition of glaucoma. Journal of Glaucoma, vol. 7, no. 3, pp. 165-69. |
| [26] | Baudouin C. (2008). Detrimental effects of preservative in eye drops: implications for the treatment of glaucoma. Acta Ophthalmol., 86(7): 716–26. |
| [27] | Bean GW & Camras CB. (2008). Commercially available prostaglandin analogs for the reduction of intraocular pressure: Similarities and differences. Surv Ophthalmol., 53(Suppl 1):S69–84. |
| [28] | Becker B, Pettit TH & Gay AJ. (1961). Topical epinephrine therapy of open angle glaucoma. Arch Ophthalmol., 66(2): 219–5. |
| [29] | Becker B. (1958). The decline in aqueous secretion and outflow facility with age. Am J Ophthalmol., 46(5):731–6. |
| [30] | Beckman H & Sugar HS. (1973). Neodymium laser cyclocoagulation. Arch Ophthalmol., 90: 27–8. |
| [31] | Beckman H, Kinoshita A, Rota AN, et al. (1972). Transscleral ruby laser irradiation of the ciliary body in the treatment of intractable glaucoma. Trans Am Acad Ophth Otol., 76:423–36. |
| [32] | Bert RJ, Caruthers SD, Jara H, et al. (2006). Demonstration of an anterior diffusional pathway for solutes in the normal human eye with high spatial resolution contrast-enhanced dynamic MR imaging. Invest Ophthalmol Vis Sci., 47: 5153–62. |
| [33] | Bill A. (1975). Blood circulation and fluid dynamics of the eye. Physiol Rev., 55(3):383–417. |
| [34] | Blondeau P, Rousseau JA. (2002). Allergic reactions to brimonidine in patients treated for glaucoma. Can J Ophthalmol., 37:21–6. |
| [35] | Bloom PA, Tsai JC, Sharma K, et al. (1997). Cyclodiode: transscleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology., 104:1508–20. |
| [36] | Boyle JE, Ghosh K, Gieser DK, et al. (1998). A dorzolamide / timolol combination given twice daily to monotherapy with timolol and dorzolamide. Ophthalmology., 105(10): 1945–51. |
| [37] | Brancato R, Carassa R, Trabucchi G. (1991). Diode laser compared with argon laser for trabeculoplasty. Am J Ophthalmol., 112:50–5. |
| [38] | Brancato R, Giovanni L, Trabucchi G, et al. (1989). Contact transscleral cyclophotocoagulation with Nd: YAG laser in uncontrolled glaucoma. Ophthalmic Surg., 20: 547–51. |
| [39] | Brandt JD, Cantor LB, Katz LJ, Batoosingh AL, Chou C, Bossowska I; Ganfort Investigators Group II. (2008). Bimatoprost/timolol fixed combination: a 3-month double masked randomised parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma., 17(3): 211–6. |
| [40] | Brandt JD, VanDenburgh AM, Chen K, Whitcup SM. (2001). Comparison of once or twice daily bimatoprost with twice daily timolol in patients with elevated IOP; a 3-month clinical trial. Ophthalmology., 108:1023–31. |
| [41] | Brasnu E, Brignole-Baudouin F, Riancho L, Guenon JM, Warnet JM, Baudouin C.(2008). In vitro effects of preservative free tafluprost and preserved latanoprost, travoprost and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res., 33(4):303–12. |
| [42] | Brubaker RF, Schoff EO, Nau CB, et al. (2001). Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am J Ophthalmol., 131: 19–24. |
| [43] | Burr J, Azuara-Blanco A, Avenell A. (2004). Medical versus surgical interventions for open angle glaucoma. Cochrane Database of Systematic Reviews, 2. Art No.: CD004399. DOI: 10.1002/14651858. CD004399.pub2. |
| [44] | Buskirk, E.M.V., Cioffi, G.A. (1992). Glaucomatous optic neuropathy. American Journal of Ophthalmology, vol. 113, no. 4, pp.447-52. |
| [45] | Butler P, Mannschreck M, Lin S, Hwang I, Alvarado J. (1995). Clinical experience with the longterm use of 1% apraclonidine. Incidence of allergic reactions. Arch Ophthalmol., 113:293–6. |
| [46] | Bylsma SS, Samples JR, Acott TS, Van Buskirk EM. (1988). Trabecular cell division after argon laser trabeculoplasty. Arch Ophthalmol., 106: 544–7. |
| [47] | Cairns JE. (1968). Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol., 66:673–9. |
| [48] | Camras CB. (1996). Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked multicentre trial in the United States. The United States Latanoprost Study Group. Ophthalmology., 103(1): 138–47. |
| [49] | Camras, C.B., Schumer, R.A., Marks, A., Lustgarten, J.S., Serle, B.J., Stjernschantz, J., Bito, Z.L., Ponds, S.M. (1992). Intraocular pressure reduction with Ph XA34, a new prostaglandins analogue, in patients with ocular hypertensions. Archives Ophthalmology, vol.110, no. 1, pp 1733-38. |
| [50] | Cantor L, Burgoyne J, Sanders S, et al. (1998). The effect of mitomycin C on Molteno implant surgery: a 1 year randomised masked prospective study. J Glaucoma., 7:240–6. |
| [51] | Cantor LB, Katz LJ, Cheng JW, et al. (2008). Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin., 24:2905–18. |
| [52] | Cantor, L.B. (2000). The evolving Pharmacotherapeutic profile of Brimonidine and 2-adrenergic agonist, after four years of continuous use. Expert Opinion on Pharmacotherapy, vol.1, no. 4, pp. 815–34. |
| [53] | Caprioli J. (2011). The Tube versus Trabeculectomy study: Why its findings may not change clinical practice. Am J Ophthalmol., 151(5): 742–4e1. |
| [54] | Carassa RG, Bettin P, Fiori M, Brancato R. (2003). Viscocanalostomy versus trabeculectomy in white adults affected by open angle glaucoma: a 2-year randomised controlled trial. Ophthalmology., 110:882–7. |
| [55] | Chen J, Cohn RA, Lin SC, et al. (1997). Endoscopic cyclophotocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol., 124:787–96. |
| [56] | Chiou AGY, Mermoud A, Jewelewicz DA. (1998). Postoperative inflammation following deep sclerectomy with collagen implant versus standard trabeculectomy. Graefes Arch Clin Exp Ophthalmol., 236: 593–6. |
| [57] | Chiselita D. (2001). Nonpenetrating deep sclerectomy versus trabeculectomy in primary open angle glaucoma surgery. Eye., 15:197–201. |
| [58] | Chowdhury, S.H., Rashid, M., Miah, M.R., Shahriar, C.S. & Tabassum, T. (2021). Effect of Skin Diseases in Modernized Life. American Journal of Dermatology and Venereology, 10(2), 13–24. doi: https://doi.org/10.5923/j.ajdv.20211002.01. |
| [59] | Chung HS, Shin DH, Bir CM, et al. (1997). Chronic use of apraclonidine decreases its moderation of post laser intraocular pressure spikes. Ophthalmology., 104:1921–5. |
| [60] | Chung PY, Schuman JS, Netland PA, et al. (1998). Five year results of a randomised prospective clinical trial of diode vs. argon laser trabeculoplasty for open angle glaucoma. Am J Ophthalmol. 1998; 126: 185–90. |
| [61] | Cilino S, Di Pace F, Casuccio A, et al. (2004). Deep sclerectomy versus punch trabeculectomy with and without phacoemulsification: a randomised clinical trial. J Glaucoma., 13:500–6. |
| [62] | Clineschmidt CM, Williams RD, Snyder E, Adamsons IA. (1998). A randomised trial in patients inadequately controlled with timolol alone comparing the dorzolamide-timolol combination to monotherapy with timolol or dorzolamide. Dorzolamide-Timolol Combination Study Group. Ophthalmology., 105(10):1952–9. |
| [63] | Cochran, W. G. (1977). Sampling techniques. 3rd Ed. New York: John Wiley & Sons. 72-86. url: https://archive.org/details/Cochran1977SamplingTechniques_201703. |
| [64] | Collaborative Normal Tension Glaucoma Study Group. (1998). Comparison of glaucomatous progression between untreated patients with normal tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol., 126:487–97. |
| [65] | Corcostegui J, Rebolleda G, Munoz-Negrete FJ. (2004). Refractive changes after phacoemulsification combined with deep sclerectomy assisted by corneal topography. J Cataract Refract Surg., 30:2391–6. |
| [66] | Costa VP, Azuara-Blanco A, Netland PA, et al. (2004). Efficacy and safety of adjunctive mitomycin C during Ahmed glaucoma valve implantation: a prospective randomised clinical trial. Ophthalmology, 111: 1071–6. |
| [67] | Crawford K, Kaufman PL. (1987). Pilocarpine antagonises prostaglandin F2 alpha-induced ocular hypotension in monkeys: evidence for enhancement of uveoscleral outflow by prostaglandin F2 alpha. Arch Ophthalmol., 105(8): 1112–6. |
| [68] | Crawley, L., Zamir, S.M., Cordeiro, M.F., & Guo, L. (2012). Clinical Options for the Reduction of elevated Intraocular pressure. Ophthalmology and Eye Diseases, 4, 43-64. |
| [69] | Cvenkel B. (2004). One-year follow up of selective laser trabeculoplasty in open angle glaucoma. Ophthalmologica., 218:20–5. |
| [70] | Dada, T., Dave, V., Mithal, N. (2009). Medical Management of glaucoma. Journal of current Glaucoma Practice, vol. 3, no. 3, pp. 13-17. |
| [71] | Dahan E, Carmichael TR. (2005). Implantation of a miniature glaucoma device under a scleral flap. J Glaucoma., 14:98–102. |
| [72] | Dahan E, Drusedau MU. (2000). Nonpenetrating filtration surgery for glaucoma: control by surgery only. J Cataract Refract Surg., 26:695–701. |
| [73] | Dahan E, Ravinet E, Ben-Simon GJ, Mermoud A. (2003). Comparison of the efficacy and longevity of nonpenetrating glaucoma surgery with and without a new nonabsorbable hydrophilic implant. Ophthalmic Surg Lasers Imaging., 34: 457–63. |
| [74] | Dailey RA, Brubaker RF, Bourne WM. (1982). The effects of timolol maleate and acetazolamide on the rate of aqueous formation in normal human subjects. Am J Ophthalmol., 93(2): 232–7. |
| [75] | Demailly P, Lavat P, Kretz G, Jeanteur-Lunel MN. (1996). Nonpenetrating deep sclerectomy (NPDS) with or without collagen device (CD) in primary open angle glaucoma: middle term retrospective study. Int Ophthalmol., 20:131–40. |
| [76] | Denis, P., Baudouin, C., Bron, A., Nordmann, J.P., Renard, J.P., Rouland, J.F., Sellem,s., Amrane, M. (2010).First-line Latanoprost therapy in ocular hypertension or open-angle glaucoma patients: a 3-month efficacy analysis stratified by initial intraocular pressure. BioMed Central Ophthalmology, vol. 10, no. 4, pp. 1-9. |
| [77] | Detry-Morel M, Muschart F, Pourjavan S. (2008). Micropulse diopde laser (810 nm) versus argon laser trabeculoplasty in the treatment of open angle glaucoma: comparative short-term safety and efficacy profile. Bull Soc Belge Ophthalmol., 308: 21–8. |
| [78] | Dickens CJ, Hguyen N, Mora JS, et al. (1995). Long-term results of non-contact transscleral neodymium: YAG cyclophotocoagulation. Ophthalmology., 102: 1777–81. |
| [79] | Diestelhorst M, Larsson LI; European Latanoprost Fixed Combination Study Group. (2004). A 12-week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension. Br J Ophthalmol., 88:199–203. |
| [80] | Docherty JR. (2010). Subtypes of functional alpha1-adrenoreceptor. Cell Mol Life Sci., 67:405–17. |
| [81] | Donello JE, Padillo EU, Webster ML, Wheeler LA, Gill DW. (2001). Alpha 2- adrenoreceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischaemia. J Pharmacol Exp Ther., 296: 216–23. |
| [82] | Dow CT, deVenecia G. (2008). Transciliary filtration (Singh filtration) with the Fugo plasma blade. Ann Ophthalmol (Skokie)., 40:8–14. |
| [83] | Drahanský, M., Kanich, O., Březinová, E. & Shinoda, K. (2016). Experiments with Optical Properties of Skin on Fingers. International Journal of Optics and Applications, 6(2), 37-46. url: http://article.sapub.org/10.5923.j.optics.20160602.03.html. doi: 10.5923/j.optics.20160602.03. |
| [84] | Drance SM, Nash PA. (1971). The dose response of human intraocular pressure to pilocarpine. Can J Ophthalmol., 6(1): 9–13. |
| [85] | DuBiner, H.B., Mroz, M., Shapiro, A. M., Dirks, M. S. (2001).A comparison of the efficacy and tolerability of Brimonidine and Latanoprost in adults with open-angle glaucoma or ocular hypertension: A three-month, multicenter, randomized, double-masked, parallel-group trial. Clinical Therapeutics, vol. 23, no. 12, pp. 1969–83. |
| [86] | Egbert PR, Fiadoyor S, Budenz DL, et al. (2001). Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open angle glaucoma. Arch Ophthalmol., 119:345–50. |
| [87] | Egrilmez S, Ates H, Nalcaci S, et al. (2004). Surgically induced corneal refractive change following glaucoma surgery: nonpenetrating trabecular surgeries versus trabeculectomy. J Cataract Refract Surg., 30:1232–9. |
| [88] | El Sayyad F, Helal M, El-Kholify H, et al. (2000). Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open angle glaucoma. Ophthalmology., 107:1671–4. |
| [89] | European Glaucoma Prevention Study Group (EGPS). (2002). Ophthalmology. 109: 1612–21. |
| [90] | European Glaucoma Society. (2008). Terminology and Guidelines for Glaucoma. 3rd edition. Savona, Italy. Dogma: 2008. |
| [91] | Fea AM, Bosone A, Rolle T, Broglialli B, Grignolo FM. (2008). Micropulse diode laser trabeculoplasty (MDLT) A Phase II clinical study with 12 months follow up. Clin Ophthalmol., 2(2): 247–52. |
| [92] | Feldman RM, Katz LJ, Spaeth GL, et al. (1991). Long-term efficacy of repeat argon laser trabeculoplasty. Ophthalmology., 98: 1061–65. |
| [93] | Fellman RL, Sullivan EK, Ratliff M, et al. (2002). Comparison of travaprost 0.0015% and 0.004% with timolol 0.5% in patients with elevated intraocular pressure: a 6-month masked multicentre trial. Ophthalmology., 109: 998–1008. |
| [94] | Figus M, Lazzeri S, Fogagnolo P, Lester M, Martinelli P, Nardi M. (2011). Supraciliary shunts in refractory glaucoma. Br J Ophthalmol., 95: 1537–41. |
| [95] | Fiscella, R. G., Green, A., Patuszynski, D. H., & Wilensky, J. (2003).Medical Therapy Cost Considerations for Glaucoma. American Journal of Ophthalmology, vol. 136, no. 1, pp. 18–25. |
| [96] | Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. (2002). The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol., 86: 238–42. |
| [97] | Francis BA, Singh K, Lin SC. (2011). Novel glaucoma procedures: A report by the American Academy of Ophthalmology. Ophthalmology., 118: 1466–80. |
| [98] | Francis VA, Minckler D, Dustin L, et al. (2008). Trabectome Study Group. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open angle glaucoma: initial results. J Cataract Refract Surg., 34:1096–103. |
| [99] | Fraser SG & Wormwald RP. (2008). Hospital episode statistics and changing trends in glaucoma surgery. Eye, 22:3–7. |
| [100] | Freidman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN. (2004). An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol., 138(Suppl 3):S19–31. |
| [101] | Fung, A.T., Reid, S.E., Jones, M.P., Healey, P.R., McCluskey, P. J., & Craig, J. C. (2007). Meta-analysis of randomized controlled trials comparing Latanoprost with Brimonidine in the treatment of Open-Angle Glaucoma, ocular hypertension or normal-tension glaucoma. British journal of ophthalmology,vol. 91, no. 1, pp. 62–8. |
| [102] | Gandolfi SA, Cimino L. (2003). Effect of bimatoprost on patients with primary open angle glaucoma or ocular hypertension who are non-responders to latanoprost. Ophthalmology, 110(3):609–14. |
| [103] | García-Sánchez J, Rouland JF, Spiegel D, Pajic B, Cunliffe I, Traverso C, Landry J. (2004). A comparison of the fixed combination of latanoprost and timolol with the unfixed combination of brimonidine and timolol in patients with elevated intraocular pressure. A six month, evaluator masked, multicentre study in Europe. Br J Ophthalmol., 88(7): 877-83. doi: 10.1136/bjo.2003.029330. PMID: 15205229; PMCID: PMC1772215. |
| [104] | Garway-Heath, D.F., Crabb, D.P., Bunce, C., Lascaratos, G., Amalfitano, F., Anand, N., Zeyen, T. G. (2015). Latanoprost for open-angle glaucoma (UKGTS): A Randomized, multicentre, placebo-controlled trial. The Lancet, vol. 385, no. 9975, pp.1295–04. |
| [105] | Gedde SJ, Heuer DK, Parrish II RK; (2010). The Tube Versus Trabeculectomy Study Group. Review of results from the tube versus trabeculectomy study. Curr Opin Ophthalmol., 21:123–8. |
| [106] | Glaucoma Laser trial Research Group. (1995). The Glaucoma Laser Trial (GLT) and Glaucoma Laser Trial Follow-up Study: 7. Results. Am J Ophthalmol., 120: 718–31. |
| [107] | Glaucoma Laser Trial Research Group. (1990). The Glaucoma Laser Trial (GLT) 2. Results of argon laser trabeculoplasty versus topical medicines. Ophthalmology., 97: 1403–3. |
| [108] | Goldberg I, Cunha-Vaz J, Jakobsen JE, et al. (2001). Comparison of topical travoprost eye drops given once daily and timolol 0.5% given twice daily in patients with open angle glaucoma or ocular hypertension. J Glaucoma., 10: 414–22. |
| [109] | Goldenfeld M, Melamed S, Simon G, Ben Simon GJ. (2009). Titanium: sapphire laser trabeculoplasty versus argon laser trabeculoplasty in patients with open angle glaucoma. Ophthalmic Surg Lasers Imaging., 40: 264–9. |
| [110] | Goni FJ. Brimonidine/Timolol fixed combination study group. (2005). 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol., 15(5): 581–90. |
| [111] | Gracner T. (2001). Intraocular pressure reduction after selective laser trabeculoplasty in primary open angle glaucoma. Collegium Antropologicum., 25(Suppl): S111–5. |
| [112] | Gracner T. (2002). Intraocular pressure response of capsular glaucoma and primary open angle glaucoma to selective Nd:YAG laser trabeculoplasty: a prospective comparative clinical trial. Eur J Ophthalmol., 12: 287–92. |
| [113] | Granero GE & Longhi MR. (2010). Promising complexes of acetazolamide for topical ocular administration. Expert Opin Drug Deliv., 7(8): 943–53. |
| [114] | Grayson DK, Camras CB, Podos SM, Lustgarten JS. (1988). Long term reduction of intraocular pressure after repeat argon laser trabeculoplasty. Am J Ophthalmol., 106: 312–21. |
| [115] | Grueb M, Rohrbach JM, Bart-Schmidt KU, et al. (2006). Transscleral diode laser contact cyclophotocoagulation as primary and secondary surgical treatment in primary open angle glaucoma and pseudoexfoliative glaucoma. Longterm clinical outcomes. Graefes Arch Clin Exp Ophthalmol., 244:1293–9. |
| [116] | Hager H. (1973). Special microsurgical interventions 2. First experiences with the argon laser apparatus 800 [in German]. Klin Monbl Augenheilkd., 162:437–50. |
| [117] | Hamard P. & Lachkar Y. (2002). Nonpenetrating filtering surgery, evolution and results. J Fr Ophthalmol., 225: 527–36. |
| [118] | Hampton C, Shields MB, Miller KN, et al. (1990). Evaluation of a protocol for transscleral Nd-YAG cyclophotocoagulation in one hundred patients. Ophthalmology., 97:910–7. |
| [119] | Hauber FA & Scherer WJ. (2002). Influence of total energy delivery on success rate after contact diode laser transscleral cyclophotocoagulation: a retrospective case review and meta-analysis. J Glaucoma., 11:329–33. |
| [120] | Hayreh SS, Podhajsky P, Zimmerman MB. (1999). Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol., 128:301–9. |
| [121] | Hedman K, Alm A. (2000). A pooled-data analysis of three randomised double masked six-month clinical studies comparing the intraocular pressure reducing effect of latanoprost and timolol. Eur J Ophthalmol., 10:95–104. |
| [122] | Heijl A, Leske MC, Bengtsson B, et al. (2002). Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol., 120: 1268–79. |
| [123] | Heijl A, Leske MC, Hyman L, Yang Z, Bengtsson B; for the EMGT Group. (2009). Intraocular pressure reduction with a fixed treatment protocol in the early manifest glaucoma trial. Ophthalmology, 116(12): 2271–6. |
| [124] | Heijl, A., Leske, M.C., Bengtsson, B., Hyman, L., Bengtsson, B., Hussein, M. (2002). Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives Ophthalmology, vol. 120, no. 10, pp 1268–79. |
| [125] | Heuer DK, Lloyd MA, Abrams DA, et al. (1992). Which is better? One or two? A randomised clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology, 99: 1512–9. |
| [126] | Higginbotham BJ, Schuman JS, Goldberg I, et al. (2002). Bimatoprost Study Group 1 and 2. One-year randomised study comparing Bimatoprost and Timolol in glaucoma and ocular hypertension. Arch Ophthalmol., 120: 1286–9. |
| [127] | Higginbotham EJ, Diestelhorst M, Pfeiffer N, et al. (2002a). The efficacy and safety of unfixed and fixed combinations of latanoprost and other antiglaucoma medications. Surv Ophthalmol., 47(Suppl 1):S133–40. |
| [128] | Hitchings RA, Grierson I. (1983). Clinicopathological correlation in eyes with failed fistulising surgery. Trans Ophth Soc U K., 103(Pt 1):84–8. |
| [129] | Hommer A. Ganfort Investigators Group I. (2007). A double masked randomised parallel comparison of a fixed combination of bimatoprost 0.03%/timolol 0.5% with non-fixed combination use in patients with glaucoma or ocular hypertension. Eur J Ophthalmol., 17(1):53–62. |
| [130] | Hong CH, Arosemena A, Zurakowski D, Ayyala RS. (2005). Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol.. 50:48–60. |
| [131] | Horsley MB. & Chen TC. (2011). The use of prostaglandin analogues in the uveitic patient. Semin Ophthalmol., 26(4–5): 285–9. |
| [132] | Hossain, M.S., Miah, M.R., Fardous, M., Ferdous, N.J., Mostofa, M.G., Hossain, S.A.M.I., Shahriar, C.S., Talukdar, M.T.H. & Ansari, M.A.S. (2021). Histopathological Study of Oral and Oropharyngeal Lesions in a Tertiary Care Hospital. Research In Cancer and Tumor, 9(1), 1–7. url: http://article.sapub.org/10.5923.j.rct.20210901.01.html. doi: https://doi.org/10.5923/j.rct.20210901.01. |
| [133] | Hoyng PF & Kitazawa Y. (2002). Medical treatment of normal tension glaucoma. Ophthalmology, 47 (Suppl 1): S116–24. |
| [134] | Hughes BA, Bacharach J, Craven ER, et al. (2005). A three-month multi centre double masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma and ocular hypertension. J Glaucoma., 14: 392–9. |
| [135] | Hutzlemann J, Owens S, Shedden A, Adamsons I, Vargas E. (1998). Comparison of the safety and efficacy of fixed combination of dorzolamide/timolol and the concomitant administration dorzolamide and timolol: a clinical equivalence study. International Clinical Equivalence Study Group. Br J Ophthalmol., 82(11)1249–53. |
| [136] | Inan, U.U., Ermis, S.S., Yucel, A., & Ozturk, F. (2003). The effects of Latanoprost and Brimonidine on blood flow velocity of the retro bulbar vessels: a 3-month clinical trial. Acta Ophthalmologica Scandinavica, vol. 81, no. 2, pp. 155–60. |
| [137] | Ishida N, Odani-Kawabata N, Shimazaki A, Hara H. (2006). Prostanoids in the therapy of glaucoma. Cardiovasc Drug Rev., 24(1):1–10. |
| [138] | Jampel HD, Bacharach J, Sheu WP, et al. (2002). Randomised clinical trial of latanoprost and unoprostone in patients with elevated intraocular pressure. Am J Ophthalmol., 134: 863–71. |
| [139] | Johnson PB, Katz LJ, Rhee DJ. (2006). Selective laser trabeculoplasty: predictive value of early intraocular pressure measurements for success at 3 months. Br J Ophthalmol., 90:741–3. |
| [140] | Johnstone, M. A. (1997). Hypertrichosis and Increased Pigmentation of Eyelashes and Adjacent Hair in the Region of the Ipsilateral Eyelids of Patients Treated with Unilateral Topical Latanoprost. American Journal of Ophthalmology, vol. 124, no. 4, pp. 544–47. |
| [141] | Jones E, Clarke J, Khaw PT. (2005). Recent advances in trabeculectomy technique. Curr Opin Ophthalmol., 16: 107–13. |
| [142] | Kaiserman, I., Kaiserman, N., Nakar, S., & Vinker, S. (2005). The Effect of Combination Pharmacotherapy on the Prescription Trends of Glaucoma Medications. Journal of Glaucoma, vol. 14, no. 2, pp. 157–60. |
| [143] | Kajiya S, Hayakawa K, Sawaguchi S. (2000). Clinical results of selective laser trabeculoplasty. Nippon Ganka Gakkai Zasshi Japonicae., 104:160–4. |
| [144] | Kalapesi FB, Coroneo MT, Hill MA. (2005). Human ganglion cells express the alpha-2 adrenergic receptor: relevance to neuroprotection. Br J Ophthalmol., 89:758–63. |
| [145] | Kano K, Kuwayama Y, Mizoue S, Ito N. (1999). Clinical results of selective laser trabeculoplasty. Nippon Ganka Gakkai Zasshi Japonicae., 103: 612–6. |
| [146] | Kass MA, Gordon MO, Gao F, et al; (2010). Ocular Hypertension Treatment Study Group. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol., 128:276–87. |
| [147] | Kass MA, Heuer DK, Higginbotham EJ, et al. (2002). The Ocular Hypertension Treatment Study: a randomised trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open angle glaucoma. Arch Ophthalmol., 120:701–13. |
| [148] | Katz, L.J. (1999). Brimonidine Tartrate 0.2% Twice Daily vs. Timolol 0.5% Twice Daily: 1-year results in Glaucoma Patients. American Journal of Ophthalmology, vol. 127, no. 1, pp. 20–6. |
| [149] | Kenigsberg PA. (2007). Changes in medical and surgical treatments of glaucoma between 1997 and 2003 in France. Eur J Ophthalmol., 17:521–7. |
| [150] | Khairy HA, Atta HR, Green FD, et al. (2005). Ultrasound biomicroscopy in deep sclerectomy. Eye, 19:555–60. |
| [151] | Khan, M.S., Hussain, T., Uddin, B., Quaium, S.M. M.A., Tanjil, Y. & Miah, M.R. (2020). Assessment of Naso- Gastric Feeding Able to Prevent Aspiration in Pneumonia, Public Health Research, 10(2), 64-70. url: http://article.sapub.org/10.5923.j.phr.20201002.04.html. doi: https://doi.org/10.5923/j.phr.20201002.04. |
| [152] | Khaw PT. (2001). Advances in glaucoma surgery: evolution of antimetabolite adjunctive therapy. J Glaucoma., 10(5 Suppl 1): S81–4. |
| [153] | Kim YJ & Moon CS. (2000). One year follow up of laser trabeculoplasty using Q-switched frequency doubled Nd-YAG laser of 532 nm wavelength. Ophthalmic Surg Lasers., 31:394–9. |
| [154] | Kingman, S. (2004). Glaucoma is second leading cause of blindness globally. Bulletin of the World Health Organization, vol. 82, no. 11, pp. 887–88. |
| [155] | Kirwan JF, Rennie C, Evans JR. (2009). Beta radiation for glaucoma surgery. Cochrane Database of Systematic Reviews, 2. Art No.: CD003433. DOI:1.1002/14651858.CD003433.pub2. |
| [156] | Klink T, Lieb W, Grehn F. (2000). Erbium-YAG laser assisted preparation of deep sclerectomy. Graefes Arch Exp Ophthalmol., 238:792–6. |
| [157] | Kobayashi H, Kobayashi K. (2007). Randomised comparison of the intraocular pressure lowering effect of phacotrabeculectomy. Ophthalmology, 114:909–14. |
| [158] | Konstas AG, Katsimpris IE, Kaltsos K, et al. (2008). Twenty-four hour efficacy of the brimonidine/timolol fixed combination versus therapy with the unfixed components. Eye, 22(11): 1391–7. |
| [159] | Konstas AG, Quaranta L, Yan DB, et al. (2011). Twenty- four hour efficacy with the dorzolamide/timolol fixed combination compared with the brinzolamide/ timolol fixed combination in primary open angle glaucoma. Eye, Sep 30. doi:10. 1038/eye.2011.239 [Epub ahead of print.] |
| [160] | Korte JM, Kaila T, Saari KM. (2002). Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eye drops. Graefes Arch Clin Exp Ophthalmol., 240(6): 430–5. |
| [161] | Kosoko O, Gaasterland DE, Pollack IP, et al. (1996). Longterm outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. Ophthalmology, 103:1294–302. |
| [162] | Kozobolis VP, Christodoulakis EV, Tzanakis N, et al. (2002). Primary deep sclerectomy versus primary deep sclerectomy with the use of mitomycin C in primary open angle glaucoma. J Glaucoma, 11:287–93. |
| [163] | Kramer TR & Noecker RJ. (2001). Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology, 108: 773–9. |
| [164] | Kramp K, Vick HP, Guthoff R. (2002). Transscleral diode laser contact cyclophotocoagulation in the treatment of different glaucomas, also as primary surgery. Graefes Arch Clin Exp Ophthalmol., 240:698–703. |
| [165] | Krasnov MM. (1964). Sinusotomy in glaucoma. Vestn Oftalmol., 77:37–41. |
| [166] | Krasnov MM. (1968). Externalisation of Schlemm’s canal in glaucoma. Br J Ophthalmol., 52:157–61. |
| [167] | Krasnov MM. (1972). Laser puncture of the anterior chamber angle in glaucoma (a preliminary report) [In Russian]. Vest Oftalmol., 3:27–31. |
| [168] | Krupin T, Liebman JN, Greenfield DS, Ritch R, Gardiner S. (2011). Low-Pressure Glaucoma Study Group. A Randomised trial of brimonidine versus timolol in preserving visual function: results from the low pressure glaucoma treatment study. Am J Ophthalmol., 151(4):671–81. |
| [169] | Lachkar Y, Neverauskiene J, Jeanteur-Lunel MN, et al. (2004). Nonpenetrating deep sclerectomy: a 6 year retrospective study. Eur J Ophthalmol., 14:26–36. |
| [170] | Lai JS, Chua JK, Tham CC, Lam DS. (2004). Five year selective laser trabeculoplasty in Chinese eyes. Clin Exp Ophthalmol., 32:368072. |
| [171] | Lai JS, Than CC, Chan JC, et al. (2005). Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for medically uncontrolled chronic angle closure glaucoma: longterm clinical outcomes. J Glaucoma, 14:114–9. |
| [172] | Lanvin MJ, Wormald RP, Migdal CS, Hitchings RA. (1990). The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol., 108(11):1543–8. |
| [173] | Lanzetta P, Menchini U, Virgili G. (1999). Immediate intraocular pressure response to selective laser trabeculoplasty. Br J Ophthalmol., 83:29–32. |
| [174] | Latina MA & Park C. (1995). Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res., 60: 359–71. |
| [175] | Latina MA, DeLeon JM. (2005). Selective laser trabeculoplasty. Ophthalmol Clin N Am., 18:409–19. |
| [176] | Latina MA, Sibayan SA, Shin DH, et al. (1998). Q-switched 532 nm Nd-YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicentre pilot clinical study. Ophthalmology, 105:2082–8. |
| [177] | LeBlanc, R.P. (1998). Twelve-month Results of an Ongoing Randomized Trial Comparing Brimonidine Tartrate 0.2% and Timolol 0.5% Given Twice Daily in Patients with Glaucoma or Ocular Hypertension. Journal of Ophthalmology, vol. 105, no. 10, pp. 1960–67. |
| [178] | Lee R. & Hutnik, CM. (2006). Projected cost comparison of selective laser trabeculoplasty versus glaucoma medication in the Ontario Health Insurance Plan. Can J Ophthalmol., 41(4): 449–56. |
| [179] | Leske MC, Heijl A, Hussein M. (2003). Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol., 121:48–56. |
| [180] | Leske MC, Heijl A, Hussein M. (2007). Early Manifest Glaucoma Trial Group. Predictors of long term progression in the Early Manifest Glaucoma Trial. Ophthalmology, 114: 1965–72. |
| [181] | Lewis RA, von Wolff K, Tetz M, et al. (2007). Canaloplasty: circumferential viscodilatation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg., 33: 1217–26. |
| [182] | Lewis RA, von Wolff K, Tetz M, et al. (2009). Canaloplasty: circumferential viscodilatation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open angle glaucoma in adults: two year interim clinical study results. J Cataract Refract Surg., 35:814–24. |
| [183] | Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. (2001). CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomised to medications or surgery. Ophthalmology, 108:1943–53. |
| [184] | Lin P, Wollstein G, Glavas IP, et al. (2004). Contact transscleral Nd: YAG laser cyclophotocoagulation: long-term outcome. Ophthalmology, 111: 2137–43. |
| [185] | Lin S. (2002). Endoscopic cyclophotocoagulation. Br J Ophthalmol., 86: 1434–8. |
| [186] | Lin SC. (2008). Endoscopic and transscleral cyclophotocoagulation for the treatment of refractory glaucoma. J Glaucoma, 17:238–47. |
| [187] | Luke C, Dietlein TS, Jacobi PC, et al. (2003). A prospective randomised trial of viscocanalostomy with and without implantation of a reticulated hyaluronic acid implant (SKGEL) in open angle glaucoma. Br J Ophthalmol., 87: 599–603. |
| [188] | Luo, M., Miao, C.Y., Chen, W., Li, H.Y., Wang, D.L., Liu, P. (2015).Comparison of Latanoprost and Brimonidine in the treatment of open angle glaucoma. International Eye Science, 15(7), 1256-58. |
| [189] | Marchini G, Marraffa M, Brunelli C, et al. (2001). Ultrasound biomicroscopy and intraocular pressure lowering mechanisms of deep sclerectomy with reticulated hyaluronic acid implant. J Cataract Refract Surg., 27: 507–17. |
| [190] | Maris PJ Jr, Ishida K, Netland PA. (2007). Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma, 16: 14–9. |
| [191] | Marquis RE, Whitson JT. (2005). Management of glaucoma: Focus on pharmacological therapy. Drugs Aging., 22(1): 1–21. |
| [192] | Martin, L. (1999).Clinical experience with Latanoprost: A retrospective study of 153 patients. Acta Ophthalmologica Scandinavica, 77, 336-39. |
| [193] | McHugh D, Marshall J, Ffytche TJ, et al. (1990). Diode laser trabeculoplasty (DLT) for primary open angle glaucoma and ocular hypertension. Br J Ophthalmol., 74:743–7. |
| [194] | McIlraith I, Strasfield M, Colev G, Hutnik CM. (2006). Selective laser trabeculoplasty as initial and adjunctive treatment for open angle glaucoma. J Glaucoma, 15:124–30. |
| [195] | Melamed S, Ben Simon GJ, Goldenfeld M, Simon G. (2009). Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: a pilot study. Arch Ophthalmol., 127: 264–9. |
| [196] | Melamed S, Ben Simon GJ, Levkovitch-Verbin H. (2003). Selective laser trabeculoplasty as a primary treatment for open angle glaucoma; a prospective non randomised pilot study. Arch Ophthalmol., 121:957–60. |
| [197] | Melamed S, Pei J, Epstein DL. (1985). Short term effect of argon laser trabeculoplasty in monkeys. Arch Ophthalmol., 103: 1546–62. |
| [198] | Melamed S, Pei J, Epstein DL. (1986). Delayed response to argon laser trabeculoplasty in monkeys: morphological and morphometric analysis. Arch Ophthalmol., 104:10778–83. |
| [199] | Mendrinos E, Mermoud A, Shaarawy T. (2008). Nonpenetrating glaucoma surgery. Surv Ophth., 53(6): 592–630. |
| [200] | Mermoud A, Schnyder CC, Sickenberg M, et al. (1999). Comparison of deep sclerectomy with collagen implant and trabeculectomy in open angle glaucoma. J Cataract Refract Surg., 25:323–31. |
| [201] | Mermoud A. (2000). Sinusotomy and deep sclerectomy. Eye, 14: 531–5. |
| [202] | Miah, M. R., Hasan, M. M. ., Parisha, J. T., Chowdhury, S. H., Sayok, A. K., & Uddin, M. B. (2023). A Unique Revolutionary Journey across the globe to discover the novel Coronavirus. International Journal of Research -GRANTHAALAYAH, 11(4), 84–100. https://doi.org/10.29121/granthaalayah.v11.i4.2023.5137. |
| [203] | Miah, M. R., Hasan, M. M., Parisa, J. T., Alam, M. S. E., Shahriar, C. S., Akhtar, F., Begum, M., Sayok, A.K., Abdullah, F., Shamsuddin, M.A.S., Rahman, A.A.M.S., Alam, M.S., Tabassum, T., Chowdhury, S.H., Sharif, M.A., Rahman, M.S., Uddin, M.B., Tamim, M.A.K., Nazim, A.Y.M., Hannan, M.A., Uddin, M.J., Uddin, MB., Ghani, M.A., Nipa, N.S., Khan, M.S., Ahmed, G., Hossain, M.S., Rashid, M.M., Beg, M.O., Samdany, A.A., Hossain, S.A.M.I., Selim, M.A., Uddin, M.F., Nazrin, M.S., Azad, M.K.H., Malik, S.U.F., Hossain, M.K. & Chowdhury, M.A.K. (2022). Impact of Oscillated Wireless Sensor Networks to Initiate Cardiac Arrest. International Journal of Internal Medicine, 11(1), 1–17. url: http://article.sapub.org/10.5923.j.ijim.20221101.01.html, doi: https://doi.org/10.5923/j.ijim.20221101.01. |
| [204] | Miah, M. R., Hasan, M. M., Parisha, J. T., Huda, M. B., Sher-E-Alam, M., Kiew Sayok, A., Rahman, M. S., Sharif, M. A., Uddin, M. B., Chowdhury, S. H., & Bhuiyan, M. A. (2023). Misuse of Advanced Satellite Technology to Accelerate Man-made Flash Floods. International Journal of Research -GRANTHAALAYAH, 11(3), 160–171. url: https://www.granthaalayahpublication.org/journals/granthaalayah/article/view/5058. |
| [205] | Miah, M. R., Sayok, A. K., Sarok, A., & Uddin, M. B. (2018). Applications of Biological Diversity Information Systems towards Conservation at Lawachara National Park in Bangladesh. Malaysian Journal of Medical and Biological Research, 5(2), 93-104. doi: https://doi.org/10.18034/mjmbr.v5i2.457. |
| [206] | Miah, M.R. (2023). Discovery of Coronavirus (book). Scientific and Academic Publishing, California, USA. 1–345 [in press]. url: http://www.sapub.org/Book/index.aspx. |
| [207] | Miah, M.R., Alam, M.S., Hasan, M.M., Parisha, J.T., Sayok, A.K., Rahman, M.S., Sharif, M.A. & Uddin, M.B. (2022). Scientific Environmental Governance to Accelerate Sustainable Biodiversity Management. Advances in Life Sciences, 11(1), 1–16. url: http://article.sapub.org/10.5923.j.als.20221101.01.html. doi: https://doi.org/10.5923/j.als.20221101.01. |
| [208] | Miah, M.R., Chowdhury, S.H., Parisha, J.T., Rashid, M.M., Hassan, M.M. & Sayok, A.K. (2023). Impact of Radiofrequency Tracking on Body Surfaces for Acute Exacerbations of Skin Disease. American Journal of Dermatology and Venereology, 12 (1), 1–9. url: http://article.sapub.org/10.5923.j.ajdv.20231201.01.html, doi: https://doi.org/10.5923/j.ajdv.20231201.01. |
| [209] | Miah, M.R., Hannan, M.A., Rahman, AAMS., Khan, M.S., Hossain, M.M., Rahman, I.T., Hossain, M.S., Shahriar, C.S., Uddin, M.B., Talukdar, M.T.H., Alam, M.S., Hossain, S.A.M.I., Samdany, A.A., Chowdhury, S.H., Sayok, A.K. (2021). Processed Radio Frequency towards Pancreas Enhancing the Deadly Diabetes Worldwide. Journal of Endocrinology Research, 3(1), 1–20. url: https://ojs.bilpublishing.com/index.php/jer/article/view/2826. doi: https://doi.org/10.30564/jer.v3i1.2826. |
| [210] | Miah, M.R., Hasan, M.M., Hannan, M.A., Parisa, J.T., Uddin, M.J., Uddin, M.B., Rahman, A.A.M.S., Hossain, S.A.M.I., Sharif, M.A., Akhtar, F., Shamsuddin, M.A.S., Alam, M.S.E., Alam, M.S., Abdullah, F., Rahman, M.S., Uddin, M. B., Shahriar, C.S., Sayok, A.K., Begum, M., Hossain, M.M., Khan, M.S., Ahmed, G., Malik, S.U.F., Samdany, A.A., Ghani, M.A., Hossain, M.S., Nazrin, M.S., Tamim, M.A.K., Selim, M.A., Talukdar, M.T.H., Chowdhury, F.T., Rashid, T.U., Nazim, A.Y.M., Rashid, M., Chowdhury, S.H. (2022). Myths about Coronavirus: A Research Defense. Global Journal of Health Science, 14(2), 63–112. url: https://ccsenet.org/journal/index.php/gjhs/article/view/0/46717. doi: https://doi.org/10.5539/gjhs.v14n2p63. |
| [211] | Miah, M.R., Hasan, M.M., Parisa, J.T., Alam, M.S., Akhtar, F., Begum, M., Shahriar, C.S., Sayok, A.K., Abdullah, F., Shamsuddin, M.A.S., Rahman, M.S., Sharif, M.A., Rahman, A.A.M.S., Alam, M.S., Uddin, M.B. and Chowdhury, S.H. (2021g). Unexpected Effects of Advanced Wireless Sensor Technology on Climate Change. World Environment, 11(2), 41–82. url: http://article.sapub.org/10.5923.j.env.20211102.01.html, doi: https://doi.org/10.5923/j.env.20211102.01. |
| [212] | Miah, M.R., Hasan, M.M., Parisha, J.T., Sayok, A.K., Alam, M.S. & Chowdhury, S.H. (2022). Issues and Challenges in Medical Jurisprudence Due to Misuse of Wireless Sensor Technology. American Journal of Medicine and Medical Sciences, 12(12), 1277–1291. url: http://article.sapub.org/10.5923.j.ajmms.20221212.23.html. doi: https://doi.org/10.5923/j.ajmms.20221212.23. |
| [213] | Miah, M.R., Hasan, M.M., Parisha, J.T., Shahriar, C.S., Sayok, A.K., Chowdhury, S.H. (2022). Adverse Global Health Impacts Due to the Proliferation of Man-Made Technological Heatwaves. Resources and Environment, 12(3), 67–75. url: http://article.sapub.org/10.5923.j.re.20221203.01.html, doi: https://doi.org/10.5923/j.re.20221203.01. |
| [214] | Miah, M.R., Hasan, M.M., Parisha, J.T., Shahriar, C.S., Sayok, A.K. & Chowdhury, S.H (2022). Towards the Misuse of Advanced Wireless Sensor Technology to Enable the Sudden Onset of ARDS. American Journal of Medicine and Medical Sciences, 12(6), 616–638. Retrieved from http://article.sapub.org/10.5923.j.ajmms.20221206.05.html, doi: https://doi.org/10.5923/j.ajmms.20221206.05. |
| [215] | Miah, M.R., Hasan, M.M., Parisha, J.T., Shahriar, C.S., Sayok, A.K., Selim, M.A. & Chowdhury, S.H. (2023). A Scientific Innovative Approach to Recovery from Dengue Fever. Public Health Research, 13(1), 1–14. url: http://article.sapub.org/10.5923.j.phr.20231301.01.html, doi: https://doi.org/10.5923/j.phr.20231301.01. |
| [216] | Miah, M.R., Hasan, MM., Parisa, J.T., Alam, MSE, Hossain, MM., Akhtar, F., Begum, M., Sayok, AK., Abdullah, F., Shamsuddin, MAS., Rahman, AAMS., Alam, MS., Chowdhury, SH. (2021). Coronavirus: A Terrible Global Democracy. International Journal of Applied Sociology, 11(2), 46–82. url: http://article.sapub.org/10.5923.j.ijas.20211102.02.html, doi: https://doi.org/10.5923/j.ijas.20211102.02. |
| [217] | Miah, M.R., Khan, M.S., Rahman, A.A.M.S., Samdany, A.A., Hannan, M.A., Chowdhury, S.H., and Sayok, A.K. (2020). Impact of Sensor Networks towards Individuals Augmenting Causes of Diabetes. International Journal of Diabetes Research, 9(2), 1–10. url: http://article.sapub.org/10.5923.j.diabetes.20200902.02.html, doi: 10.5923/j.diabetes.20200902. |
| [218] | Miah, M.R., Miah, M.R., Mustaffa, M.S., Jayos, S., Ibrahim, N.H., Bujang, S., Saili, J. & Sayok, A.K. (2019). Towards Stimulating Tools for Advancement of Environmental Conservation through Promoting of Psychological Instruments. Journal of Sustainable Development, 12(4), 196-224. https://doi.org/10.5539/jsd.v12n4p196. Retrieved from https://www.ccsenet.org/journal/index.php/jsd/article/view/0/40313. |
| [219] | Miah, M.R., Mustaffa, M.S., Sabil, S., Madihie, A., Saili, J. & Sayok, A.K. (2018). Towards Dynamic Policy for Early Childhood Development Enhanced the Growth of Self-Regulations. International Journal of Engineering & Technology, 7 (3.30), 251–255. doi: https://doi.org/10.14419/ijet.v7i3.30.18251. |
| [220] | Miah, M.R., Rahman, A.A.M.S., Khan, M.S., Hannan, M.A., Hossain, M.S., Shahriar, C.S., Hossain, S.A.M.I., Talukdar, M.T.H., Samdany, A.A., Alam, M.S., Uddin, M.B., Sayok, A.K., and Chowdhury, S.H. (2021). Effect of Corona Virus Worldwide through Misusing of Wireless Sensor Networks. American Journal of Bioinformatics Research, 11(1), 1–31. url: http://article.sapub.org/10.5923.j.bioinformatics.20211101.01.html. doi: https://doi.org/10.5923/j.bioinformatics.20211101.01. |
| [221] | Miah, M.R., Rahman, A.A.M.S., Khan, M.S., Samdany, A.A., Hannan, M.A., Chowdhury, S.H., Sayok, A.K. (2020). Impact of Sensor Technology Enhancing Corona Disease. American Journal of Biomedical Engineering, 10 (1), 1–11. url: http://article.sapub.org/10.5923.j.ajbe.20201001.03.html, doi: https://doi.org/10.5923/j.ajbe.20201002. |
| [222] | Miah, M.R., Rahman, A.A.M.S., Parisa, J.T., Hannan, M.A., Khan, M.S., Samdany, A.A., Sayok, A.K. and Chowdhury, S.H. (2021d). Discovery of Coronavirus with Innovative Technology. Science and Technology, 11(1), 7–29. url: http://article.sapub.org/10.5923.j.scit.20211101.02.html, doi: https://doi.org/10.5923/j.scit.20211101.02. |
| [223] | Miah, M.R., Rahman, A.A.M.S., Samdany, A.A., & Chowdhury, S.H. (2021a). A Dynamic Scientific Model for Recovery of Corona Disease. Frontiers in Science, 11(1), 1–17. url: http://article.sapub.org/10.5923.j.fs.20211101.01.html. doi: https://doi.org/10.5923/j.fs.20211101.01 |
| [224] | Miah, M.R., Rahman, A.A.M.S., Sayok, A.K., Samdany, A.A., and Hannan, M.A. (2021). How to fight the COVID-19 global crisis? World Journal of Environmental Research, 11(2), 31–38. doi: https://doi.org/10.18844/wjer.v11i2.5855. |
| [225] | Miah, M.R., Rahman, AAMS., Hasan, M.M., Parisa, J.T., Hannan, M.A., Hossain, M.M., Alam, M.S., Alam, M.S.E., Akhtar, F., Ghani, M.A., Khan, M.S., Shahriar, C.S., Sayok, A.K., Begum, M., Malik, S.U.F., Samdany, A.A., Ahmed, G. and Chowdhury, S.H. (2021c). Adverse Effects of Wireless Sensor Technology to Debilitating in Numbness. International Journal of Virology and Molecular Biology, 10(1), 12–25. url: http://article.sapub.org/10.5923.j.ijvmb.20211001.03.html, doi: https://doi.org/10.5923/j.ijvmb.20211001.03. |
| [226] | Miah, M.R., Sayok, A.K., Rahman, AAMS, Samdany, A.A., Akhtar, F., Azad, A.K., Hasan, MM, Khan, M.S., Alam, S.E., Alam, MS., Uddin, M.B., Abdullah, F., Shahriar, C.S., Shamsuddin, MAS., Uddin, M.B., Sarok, A., Rahman, IT., Chowdhury, SC., Begum, M. (2021e). Impact of Sensor Networks on Aquatic Biodiversity in Wetland: An Innovative Approach, Geosciences, 11(1), 10–42. url: http://article.sapub.org/10.5923.j.geo.20211101.02.html, doi: https://doi.org/10.5923/j.geo.20211101.02. |
| [227] | Miah, M.R., Uddin, M.M., Parisha, J.T., Shahriar, C.S., Alam, M.S., Chowdhury, S.H., Nazim, A.Y.M., Hannan, M.A., Uddin, M.J., Uddin, M.B., Nipa, N.S., Khan, M.S., Ahmed, G., Hossain, M.S., Rashid, M.M., Samdany, A.A., Hossain, S.A.M.I., Selim, M.A., Uddin, M.F., Nazrin, M.S., Azad, MKH., Malik, SUF., Hossain, M.M., Chowdhury, M.A.K., Tanjil, Y., Talukdar, MTH., Rahman, AAMS., Sayok, A.K., Sharif, M., A., Rahman, MS., Hasan, M.M., Alam, M.S., Uddin, M.B., Patowary, D., Bhuiyan, MRA. & Chowdhury, MTR. (2023b). Uncontrolled Advanced Wireless Sensor Technology to Enable Early Growth of Stomach Cancer. American Journal of Stem Cell Research, 5(1), 8–39. url: http://article.sapub.org/10.5923.j.ajscr.20230501.02.html, doi: https://doi.org/10.5923/j.ajscr.20230501.02. |
| [228] | Miah, M. R. (2018). Assessment of Environmental Policy Instruments along with Information Systems for Biodiversity Conservation in Bangladesh (Doctoral dissertation), PhD Thesis. IBEC, UNIMAS, Malaysia. 1-480. Retrieved from https://ir.unimas.my/id/eprint/24535/. |
| [229] | Mielke C, Dawda VK, Anand N. (2006). Deep sclerectomy and low dose mitomycin C: a randomised prospective trial in West Africa. Br J Ophthalmol. 2006; 90: 310–3. |
| [230] | Migdal C, Gregory W, Hitchings R. (1994). Long term functional outcome after early surgery compared with laser and medicine in open angle glaucoma. Ophthalmology, 101:1651–6. |
| [231] | Migdal C, Hitchings R. (1986). Control of chronic simple glaucoma with primary medical, surgical and laser treatment. Trans Ophthalmol Soc U K., 105(Pt 6):653–6. |
| [232] | Minckler DS, Baerveldt G, Alfaro MR, Francis BA. (2005). Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology, 112:962–7. |
| [233] | Minckler DS, Baerveldt G, Ramirez MA, et al. (2006). Clinical results with the Trabectome, a novel surgical device for treatment of open angle glaucoma. Trans Am Ophthalmol Soc., 104:40–50. |
| [234] | Minckler DS, Francis BA, Hodapp EA, et al. (2003). Aqueous shunts in Glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology, 115: 1089–98. |
| [235] | Minckler DS, Shammas A, Wilcox M, Ogden TE. (1987). Experimental studies of aqueous filtration using the Molteno implant. Trans Am Ophthalmol Soc., 85:368–92. |
| [236] | Minckler DS, Vedula SS, Li TJ, et al. (2006). Aqueous shunts for glaucoma. Cochrane Database of Systematic Reviews, 2. Art. No.: CD004918. DOI:10.1002/14651858. |
| [237] | Mishima, H.K., Masuda, K., Kitazawa, Y., Azuma, I., Araie, M. (1996). A Comparison of Latanoprost and Timolol in Primary Open-angle Glaucoma and Ocular Hypertension. Archives Ophthalmology, 114(8), 929-32. |
| [238] | Monemi, S., Spaeth, G., DaSilva, A. Popincalk, S., Ilitchev, E., Liebmann, J., Ritch, R., Heon, S., Crick, R. P., Cfild, A., & Sartazai, M. (2005). Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Human Molecular Genetics, 14(6), 725–33. |
| [239] | Moriarty AP, McHugh JD, Ffytche TJ, et al. (1993). Longterm follow up of diode laser trabeculoplasty for primary open angle glaucoma and ocular hypertension. Ophthalmology, 100:1614–8. |
| [240] | Murphy CC, Burnett CA, Spry PG, et al. (2003). A two centre study of the dose response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol., 87:1252–7. |
| [241] | Musch, D.C., Gillespie, B.W., Niziol, L.M., Cashwell, L.F., Lichter, P.R., & Collaborative Initial Glaucoma Treatment Study Group. (2007). Factors associated with intraocular pressure before and during 9 years of treatment in the Collaborative Initial Glaucoma Treatment Study. Journal of Ophthalmology, 115(6), 927–33. |
| [242] | Netland PA, Landry T, Sullivan EK, et al. (2001). Travoprost compared with latanoprost and timolol in patients with open angle glaucoma or ocular hypertension. Am J Ophthalmol., 132(4):472–84. |
| [243] | Neurdorfer M, Sadetzki S, Anisimova S, Geyer O.(2004). Nonpenetrating deep sclerectomy with the use of adjunctive mitomycin C. Ophthalmic Surg Lasers Imaging., 35:6–12. |
| [244] | Noecker RS, Dirk MS, Choplin NT, Bernstein P, Batoosingh AL, Whitcup SM; for the Bimatoprost/Latanoprost Study Group. (2003). A six-month randomised clinical trial comparing the IOP lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J ophthalmol., 135(1):55–63. |
| [245] | Nordlund, J.R., Pasquale, L.R., Robin, A.L., Rudikoff, M.T., Ordman, J., Chen, K.S. (1995). The Cardiovascular, Pulmonary and Ocular Hypotensive Effects of 0.2% Brimonidine. Archives Ophthalmology, 113, 77- 83. |
| [246] | Nordman JP, Mertz B, Yannoulis NC, et al. (2002). A double masked randomised comparison of the efficacy and safety of unoprostone with timolol and betaxolol in patients with primary open angle glaucoma including pseudoexfoliation glaucoma or ocular hypertension. 6 months data. Am J Ophthalmol., 133:1–10. |
| [247] | Nordstrom, B. L., Friedman, D. S., Mozaffari, E., Quigley, H. A., & Walker, A. M. (2005). Persistence and Adherence with Topical Glaucoma Therapy. American Journal of Ophthalmology, 140(4), 598–11. |
| [248] | Noureddin BN, Wilson-Holt N, Lavin M, et al. (1992). Advanced uncontrolled glaucoma: Nd: YAG cyclophotocoagulation or tube surgery. Ophthalmology., 99: 430–7. |
| [249] | Noureddin BN, Zein W, Haddad C, et al. (2006). Diode laser transscleral cyclophotocoagulation for refractory glaucoma: a 1 year follow up of patients treated using an aggressive protocol. Eye, 20:329–35. |
| [250] | O’Brart DP, Rowlands E, Islam N, Noury AM. (2002). A randomised prospective study comparing trabeculectomy augmented with antimetabolites with a viscocanalstomy technique for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol., 86: 748–54. |
| [251] | O’Brart DP, Shiew M, Edmunds B. (2002a). A randomised prospective study comparing trabeculectomy with viscocanalostomy with adjunctive antimetabolite usage for the management of open angle glaucoma uncontrolled by medical management. Br J Ophthalmol., 88:1012–7. |
| [252] | Ocklind, A. (1998). Effect of Latanoprost on the Extracellular Matrix of the Ciliary Muscle. A Study on Cultured Cells and Tissue Sections. Experimental Eye Research, 67(2), 179–91. |
| [253] | Palmer SS. (1991). Mitomycin as an adjunct chemotherapy with trabeculectomy. Ophthalmology, 98(3): 317–21. |
| [254] | Panda, S. (2023, March 13). Glaucoma Week 2023: 90 percent of cases are undiagnosed in India. Health Care. Financial Express, India. Retrieved from https://www.financialexpress.com/healthcare/news-healthcare/glaucoma-week-2023-90-percent-of-glaucoma-cases-are-undiagnosed-in-india/3007074/ on July 3, 2023 at 7:00 pm. |
| [255] | Parisha, J.T., Miah, M.R., Hasan, M.M., & Begum, M. (2022). Impact of Environmental Pollution along with Technology for Conserving of Biodiversity. International Journal of Ecosystem, 12(1), 20–30. url: http://article.sapub.org/10.5923.j.ije.20221201.02.html, doi: https://doi.org/10.5923/j.ije.20221201.02. |
| [256] | Park, B.C., Tibudan, M., Samaraweera, M., Shen, X., & Yue, B. Y. J. T. (2007). Interaction between two glaucoma genes, optineurin and myocilin.Genes to Cells, 12(8), 969–79. |
| [257] | Parrish RK, Palmberg P, Sheu WP; for the XLT Study Group. (2003). A comparison of latanoprost, bimatoprost and travoprost in patients with elevated intraocular pressure: A 12-week randomised masked-evaluator multicentre study. Am J Ophthalmol., 135(5): 688–703. |
| [258] | Patel, S.S. & Spencer, C.M. (1996) Latanoprost. A review of its pharmacological properties, clinical efficacy and tolerability in the management of primary open-angle glaucoma and ocular hypertension. Drugs & Aging, 9(5), 363–78. |
| [259] | Pucci V, Tappainer F, Borin S, et al. (2003). Long-term follow up after transscleral diode laser photocoagulation in refractory glaucoma. Ophthalmologica., 217:279–83. |
| [260] | Quigley HA. (2011). Glaucoma. Lancet, 377:1367–77. |
| [261] | Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL. (2007). Utilisation of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology, 114:2265–70. |
| [262] | Ravinet E, Tritten JJ, Roy S, et al. (2002). Descemet membrane detachment after nonpenetrating filtering surgery. J Glaucoma, 11:244–52. |
| [263] | Richter CU, Shingleton BJ, Bellows Ar, et al. (1987). Retreatment with argon laser trabeculoplasty. Ophthalmology, 94:1085–9. |
| [264] | Rivier D, Roy S, Mermoud A. (2007). Ex-PRESS R-50 miniature glaucoma implant insertion under the conjunctiva combined with cataract extraction. J Cataract Refract Surg., 33:1946–52. |
| [265] | Robin AL. (1988). Short term effects of unilateral 1% apraclonidine therapy. Arch Ophthalmol., 106(7): 912–5. |
| [266] | Rolim de Moura CR, Paranhos A Jr, Wormald R. (2007). Laser trabeculoplasty for open angle glaucoma. Cochrane Database of Systematic Reviews, 4 Art No.: CD003910. DOI:10.1002/12651858.CD003919.pub2. |
| [267] | Rosenberg LF, Krupin T, Ruderman J, et al. (1995). Apraclonidine and anterior segment laser surgery: Comparison of 0.5% vs. 1.0% apraclonidine for prevention of postoperative intraocular pressure rise. Ophthalmology, 102: 1312–8. |
| [268] | Rozsival P, Kana V, Hovorkova M. (2004). Selective laser trabeculoplasty. Ceska a Slovenska. Oftalmologie, 60: 267–74. |
| [269] | Sall KN, Greff LJ, Johnson-Pratt LR, et al. (2003). Dorzolamide/timolol 51 combination versus concomitant administration of brimonidine and timolol: a six-month comparison of efficacy and tolerability. Ophthalmology, 110(3): 615–24. |
| [270] | Samples JR, Singh K, Lin SC, et al. (2011). Laser trabeculoplasty for open angle glaucoma; A report by the American Academy of Ophthalmology. Ophthalmology, 118(11): 2296-302. doi: 10.1016/j.ophtha.2011.04.037. Epub 2011 Aug 17. PMID: 21849211. |
| [271] | Sarodia U, Shaarawy T, Barton K. (2007). Nonpenetrating glaucoma surgery: a critical evaluation. Curr Opin Ophthalmol., 18:152–8. |
| [272] | Schlect LP. & Brubaker RF. (1998). The effects of withdrawal of timolol in chronically treated glaucoma patients. Ophthalmology, 95(9):1212–6. |
| [273] | Schuman JS, Bellows AR, Shingleton BJ, et al. (1992). Contact transscleral Nd:YAG laser cyclophotocoagulation: midterm results. Ophthalmology, 99:1089–95. |
| [274] | Schuman JS, Puliafito CA, Allingham RR, et al. (1990). Contact transscleral continuous wave neodymium: YAG laser cyclophotocoagulation. Ophthalmology, 97: 571–80. |
| [275] | Schuman JS. (1996). Clinical experience with brimonidine 0.2% and timolol 0.5% in glaucoma and ocular hypertension. Surv Ophthalmol., 41(Supp1): S27–37. |
| [276] | Schuman JS. Horowitz B, Choplin NT, et al. (1997). A 1 year study of brimonidine twice daily in glaucoma and ocular hypertension: a controlled randomised multicentre clinical trial. Chronic Brimonidine Study Group. Arch Ophthalmol., 115(7): 847–52. |
| [277] | Schuman, J.S. (2000). Effects of systemic beta-blocker therapy on the efficacy and safety of topical Brimonidine and Timolol. Journal of Ophthalmology, 107(6), 1171-77. |
| [278] | Serle, J.B. & The Brimonidine Study Group-iii (1996). A comparison of safety and efficacy twice-daily Brimonidine 0.2% VS Betoxolol 0.25% in subjects with elevated intraocular pressure. Survey of Ophthalmology, 41, 39-47. |
| [279] | Shaarawy T, Nguyen C, Schnyder C, Mermoud A. (2004). Comparative study between deep sclerectomy with and without collagen implant: long term follow up. Br J Ophthalmol., 88:95–8. |
| [280] | Shaarawy T. & Mermoud A. (2005). Deep sclerectomy in one eye versus deep sclerectomy with implant in the contralateral eye of the same patient: long term follow up. Eye, 19:298–302. |
| [281] | Shah N, Yadav R, Nagar M. (2006). Selective laser trabeculoplasty: the effect of enhancement and retreatment on IOP control. Paper presented at the XXIV Congress of the European Cataract and Refractive Surgeons (ESCRS) London; 2006. |
| [282] | Shahsuvaryan, M.L. (2013). Glaucomatous Optic Neuropathy Management: The Role of Neuroprotective Agents. Medical Hypothesis & Innovation Ophthalmology Journal, 2(2), 42-46. |
| [283] | Sharif, N.A., Kelly, C.R., Crider, J.Y. (2003) Human Trabecular meshwork cell responses induced by Bimatoprost, Travoprost, Unoprostone, and other FP Prostaglandin receptor agonist analogues. Investigative Ophthalmology& Visual Science, 44, 715–21. |
| [284] | Shaya, F.T., Mullins, C.D., Wong, W. & Cho, j. (2002). Discontinuation rates of topical glaucoma medications in a managed care Population. The American journal of managed Care, 8(10), 271–77. |
| [285] | Sherwood M. & Brandt J. (2001). Six-month comparison of bimatoprost once daily and twice daily with timolol twice daily in patients with elevated intraocular pressure. Surv Ophthalmol., 45(Suppl 4):S361–8. |
| [286] | Sherwood MB, Craven ER, Chou C, et al. (2006). Twice daily 0.2% brimonidine0.5% timolol fixed combination therapy vs. monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomised trial. Arch Ophthalmol., 124(9):1230–8. |
| [287] | Shields MB. & Shields SE. (1994). Noncontact transscleral Nd:YAG cyclophotocoagulation: a long term follow up of 500 patients. Trans Am Ophthalmol Soc., 92:271–87. |
| [288] | Shingleton B, Tetz M, Korber N. (2008). Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one year results. J Cataract Refract Surg., 34: 433–40. |
| [289] | Simmons RB, Shields MB, Blasini M, et al. (1991). Transcleral Nd: YAG laser cyclophotocoagulation with a contact lens. Am J Ophthalmol., 112: 671–7. |
| [290] | Singh D, Bundela R, Agarwal A, et al. (2002). Goniotomy ab interno “a glaucoma filtering surgery” using the Fugo Plasma Blade. Ann Ophthalmol (Skokie), 34:183–7. |
| [291] | Singh D. & Singh K. (2002a). Transciliary filtration usinf the Fugo Blade. Ann Ophthalmol (Skokie), 34:183–7. |
| [292] | Song J, Lee PP, Epstein DL, et al. (2005). High failure rate associated with 180 degrees selective laser trabeculoplasty. J Glaucoma, 14:400–8. |
| [293] | Spencer AF. & Vernon SA. (1999). Cyclodiode: results of a standard protocol. Br J Ophthalmol., 83:311–6. |
| [294] | Spiegel D, Garcia-Feijoo J, Garcia-Sanchez J, Lamielle H. (2008). Coexistent primary open angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent and concurrent cataract surgery. Adv Ther., 25:453–64. |
| [295] | Spiegel D, Wetzel W, Haffner DS, Hill RA. (2007). Initial clinical experience with the trabecular micro-bypass stent in patients with glaucoma. Adv Ther., 24:161–70. |
| [296] | Spiegel D, Wetzel W, Neuhann T, et al. (2009). Coexistent primary open angle glaucoma and cataract: interim analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol., 19:393–9. |
| [297] | Stamper RL, Wigginton SA, Higginbottom EJ. (2002). Primary drug treatment for glaucoma: Beta blockers versus other medications. Surv Ophthalmol., 47(1):63–73. |
| [298] | Starita RJ, Fellman RL, Spaeth GL, et al. (1984). The effect of repeating full circumference argon laser trabeculoplasty. Ophthalmic Surg., 15:41–3. |
| [299] | Stegmann R, Pienaar A, Miller D. (1999). Viscocanalostomy for open angle glaucoma in black African patients. J Cataract Refract Surg., 25:316–22. |
| [300] | Stein JD. & Challa P. (2007). Mechanisms of action and efficacy of argon laser trabeculoplasty and selective laser trabeculoplasty. Curr Opin Ophthalmol., 18:140–5. |
| [301] | Stewart, W.C., Day, D.G., Stewart, J.A., Schuhr, J., & Latham, K. E. (2001). The efficacy and safety of Latanoprost 0.005% once daily versus Brimonidine 0.2% twice daily in open-angle glaucoma or ocular hypertension. American Journal of Ophthalmology, 131 (5), 631–35. |
| [302] | Stewart, W. C., Hudgins, A.C., Pruitt, C.A., Sine, C. (1999). Daily cost of newer glaucoma agents. Journal of Ocular Pharmacology, 15(5), 379-88. |
| [303] | Strutton DR. & Walt JG. (2004). Trends in glaucoma surgery before and after the introduction of new topical glaucoma pharmacotherapies. J Glaucoma, 13: 221–6. |
| [304] | Sunaric-Megevand G, Leuenberger P. (2001). Results of viscocanalostomy for primary open angle glaucoma. Am J Ophthalmol., 132:221–8. |
| [305] | Susanna R Jr, Chew P, Kitazawa Y. (2002). Current status of prostaglandin therapy: latanoprost and unoprostone. Surv Ophthalmol., 47(Suppl 1): S97–104. |
| [306] | Susanna R Jr, Giampani J Jr, Borges AS, et al. (2001). A double masked randomised clinical trial comparing latanoprost with unoprostone in patients with open angle glaucoma or ocular hypertension. Ophthalmology, 108: 259–63. |
| [307] | Susanna, R., & Medeiros, F.A. (2001). The pros and cons of different Prostanoids in the medical management of glaucoma. Current Opinion in Ophthalmology, 12, 149–56. |
| [308] | Sutton A, Gilvarry A, Ropo A. (2007). A comparative placebo-controlled study of prostanoid fluoroprostaglandin receptor agonists tafluprost and latanprost in healthy males. J Ocul Pharmacol Ther., 23(4): 359–65. |
| [309] | Sutton A, Gouws P, Ropo A. (2008). Tafluprost, a new potent prostanoid FP- receptor agonist: a dose response study on pharmacodynamics and tolerability in healthy volunteers. Int J Clin Pharmacol Ther., 46(8): 400–6. |
| [310] | Takagi Y, Nakajima T, Shimazaki A, et al. (2004). Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res., 78(4): 767–76. |
| [311] | Tamm ER, Carassa RG, Albert DM, et al. (2004). Viscocanalostomy in rhesus monkeys. Arch Ophthalmol., 122:1826–38. |
| [312] | Tamura, H., Kawakami, H., Kanamoto, T., Kato,T., Yokoyama, T., Sasaki, K., Izumi, Y., Matsumoto, M., Mishima, H. K. (2006). High frequency of Open-Angle Glaucoma in Japanese patients with Alzheimer’s disease. Journal of the Neurological Sciences, 246, 79–83. |
| [313] | Thomas, R., Parikh, R., Muliyi, J., George, R., Paul, P., Abraham, L.M. (2003).Comparison between Latanoprost and Brimonidine efficacy and safety in Indian eyes. Indian Journal of Ophthalmology, .51(2), 123-128. |
| [314] | Toris CB, Camras CB, Yablonski ME, Brubaker RF. (1997). Effects of exogenous prostaglandins on aqueous humor dynamics and blood-aqueous barrier function. Surv Ophthalmol., 41(Suppl 2): S69–75. |
| [315] | Toris CB, Gabelt BT, Kaufman PL. (2008). Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol., 53 (Suppl 1): S107–20. |
| [316] | Toris CB. & Pederson JE. (1987). Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci., 28(3): 477–81. |
| [317] | Toris, C. B., Camras, C. B., & Yablonski, M. E. (1999). Acute versus chronic effects of Brimonidine on Aqueous Humor Dynamics in Ocular Hypertensive Patients. American Journal of Ophthalmology, 128(1), 8–14. |
| [318] | Toris, C. B., Gleason, M.L., Camras, C.B., Yablonski, M.E. (1995) Effects of Brimonidine on Aqueous Humor Dynamics in Human Eyes. Archives Ophthalmology, vol. 113, pp.1514-17. |
| [319] | Traverso CE, De Feo F, Messas-Kaplan A, et al. (2005). Long term effect on IOP of a stainless steel glaucoma drainage implant (Ex-PRESS) in combined surgery with phacoemulsification. Br J Ophthalmol., 89:425–9. |
| [320] | Tsukamoto H, Yokoyama T, Okada K, et al. (2000). Substituting latanoprost (Xalatan) for isopropyl unoprostone (Rescula) in monotherapy and combination therapy. Acta Ophthalmol Scand., 78:604–5. |
| [321] | Uddin, M.B., Hoque, M., Ali, M.M., Islam, A. & Miah, M.R. (2021). Abandonment and Outcome of Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia. Research In Cancer and Tumor, 9(1), 8–14. url: http://article.sapub.org/10.5923.j.rct.20210901.02.html. doi: https://doi.org/10.5923/j.rct.20210901.02. |
| [322] | Van Buskirk EM, Pond V, Rosenquist RC, Acott TS. (1984). Argon laser trabeculoplasty: studies of mechanism of action. Ophthalmology, 91: 1005–10. |
| [323] | Van der Valk R, Schouoten JS, Webers CA, et al. (2005). The impact of a nationwide introduction of new drugs and a treatment protocol for glaucoma on the number of glaucoma surgeries. J Glaucoma, 14:239–42. |
| [324] | van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. (2005). Intraocular pressure lowering effects of all commonly used glaucoma drugs. Ophthalmology, 112:1177–85. |
| [325] | Vold, S.D., Riggs, W.L., Jackimiec, J. (2002). Cost Analysis of Glaucoma Medications: A 3-year Review. Journal of Glaucoma, 11(4), 354-58. |
| [326] | Waewar RE, Bullock JD, Ballal D. (1998). Cystoid macular oedema and anterior uveitis associated with latanoprost use. Ophthalmology, 105: 263–8. |
| [327] | Walters, T.R. (1996) Development and use of Brimonidine in Treating Acute and Chronic Elevations of Intraocular Pressure: A Review of Safety, Efficacy, Dose Response, and Dosing Studies. Survey of Ophthalmology, 41(1), 19-26. |
| [328] | Wamsley S, Moster MR, Rai S, et al. (2004). Results of the use of the ExPRESS miniature glaucoma implant in technically challenging, advanced glaucoma cases: a clinical pilot study. Am J Ophthalmol., 38: 1049–51. |
| [329] | Wan Z, Woodward DF, Cornell CL, et al. (2007). Bimatoprost prostamide activity and conventional drainage. Invest Ophthalmol Vis Sci., 48: 4107–15. |
| [330] | Wand M, Gilbert CM, Liesegang TJ. (1999). Latanoprost and herpes simplex keratitis. Am J Ophthalmol., 127:602–4. |
| [331] | Wand, M., Ritch, R., Isbey, E.K., Zimmerman, T.J. (2001). Latanoprost and Periocular Skin Color Changes. Archives Ophthalmology, 119(4), 611–19. |
| [332] | Warwar RE, Bullock JD. (1999). Latanoprost-induced uveitis. Surv Ophthalmol., 43(5): 466-8. PMID: 10340564. |
| [333] | Watson P, Stjernschantz J. (1996). A six-month randomised double blind study comparing the effects of latanoprost and timolol on primary open angle glaucoma and ocular hypertension. The Latanoprost Study Group. Ophthalmology, 103(1): 126–37. |
| [334] | Weber PA, Burton GD, Epitropoulos AT. (1989). Laser trabeculoplasty retreatment. Ophthalmic Surg., 20:702–6. |
| [335] | Webers CA, Becjers HJ, Zeegers MP, Nuijts RM, Hendrikse F, Schouten JS. (2010). The intraocular pressure lowering effect of prostaglandin analogues combined with topical β blocker therapy: a systematic review and meta-analysis. Ophthalmology, 117(11): 2067–74. |
| [336] | Weinreb RN, Toris CB, Gabelt BT, et al. (2002). Effects of prostaglandins on aqueous humor outflow pathways. Surv Ophthalmol., 47(Suppl 1): S53–64. |
| [337] | Weinreb RN. (2000). Uveoscleral outflow: the other outflow pathway. J Glaucoma. Oct 2000; 9(5): 343–5. |
| [338] | Whitcup SM, Cantor LB, VanDenburgh AM, Chen K. (2003). A randomised double masked multicentre clinical trial comparing bimatoprost and timolol for the treatment of glaucoma and ocular hypertension. Br J Ophthalmol., 87: 57–62. |
| [339] | Wild GJ, Kent AR, Peng Q. (2001). Dilation of Schlemm’s canal in viscocanalostomy: comparison of 2 viscoelastic substances. J Cataract Refract Surg., 27:1294–7. |
| [340] | Wilkins M, Indar A, Wormald R. (2005). Intraoperative Mitomycin C for glaucoma surgery. Cochrane Database of Systematic Reviews, 4. Art No.: CD002897. DOI: 10.1002/14651858.CD002897.pub.2. |
| [341] | Wilmsmeyer S, Philippin H, Funk J. (2006). Excimer laser trabeculotomy: a new, minimally invasive procedure for patients with glaucoma. Graefes Arch Clin Exp Ophthalmol., 244:670–6. |
| [342] | Wise JB, Witter SL. (1979). Argon laser therapy for open angle glaucoma: a pilot study. Arch Ophthalmol., 97: 319–22. |
| [343] | Wistrand PJ, Stjernschantz J, Olsson K. (1997). The incidence and time-course of latanoprost induced iridial pigmentation as a function of eye colour. Surv Ophthalmol., 41(Suppl 2):S129–38. |
| [344] | Wistrand, P. J., Stjernschantz, J., & Olsson, K. (1997). The incidence and time-Course of Latanoprost-Induced Iridial Pigmentation as a Function of Eye Color. Survey of Ophthalmology, 41(2), 129–38. |
| [345] | Wormald R, Wilkins M, Bunce C. (2001). Postoperative 5-Fluorouracil for glaucoma surgery. Cochrane Database of Systematic Reviews, 3. Art. No.: CD001132. DOI:10.1002/14651858.CD001132. |
| [346] | Wright MM, Grajewski AI, Feuer WJ. (1991). YAG cyclophotocoagulation: outcome of treatment for uncontrolled glaucoma. Ophthalmic Surg., 22: 279–83. |
| [347] | Yalvac IS, Sahin M, Eksioglu U, et al. (2004). Primary viscocanalostomy versus trabeculectomy for primary open angle glaucoma: three year prospective randomised clinical trial. J Cataract Refract Surg., 30:2050–7. |
| [348] | Yamagishi R, Aihara M, Araie M. (2011). Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp Eye Res., 93: 265–70. |
| [349] | Yamamoto T, Kitazawa Y. (1997). Iris colour change developed after topical isopropyl unoprostone treatment. J Glaucoma, 6:430–2. |
| [350] | Yarangumeli A, Gurboz Koz O, Alp MN, et al. (2005). Viscocanalostomy with mitomycin C: a preliminary study. Eur J Ophthalmol., 15:202–8. |
| [351] | Yoles E, Wheeler LA, Scwartz M. (1999). Alpha2- adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest Ophthalmol Vis Sci., 40:65–73. |
| [352] | Youn J, Cox TA, Herndon LW, et al. (1998). A clinical comparison of transscleral diode laser contact cyclophotocoagulation with Nd: YAG and semiconductor diode lasers. Am J Ophthalmol., 126:640–7. |
| [353] | Ziai N, Dolan JW, Kacere RD, Brubaker RF. (1993). The effects on aqueous dynamics of PhXA41, a new prostaglandin F2α analogue, after topical application in normal and ocular hypertensive human eyes. Arch Ophthalmol., 111: 1351–8. |
| [354] | Martin, L. (1999). Clinical experience with Latanoprost: A retrospective study of 153 patients. Acta Ophthalmologica Scandinavica, 77, 336-39. |
| [355] | The Tesla Space. (2023). How Neuralink will cure blindness. url: https://www.youtube.com/watch?v=mV69oJsNyrs (Retrieved on July 4, 2023 at 9:00 pm). |


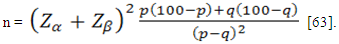
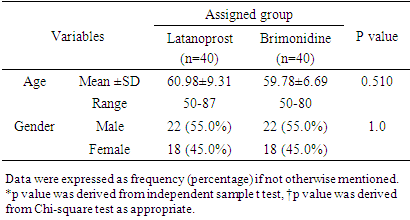
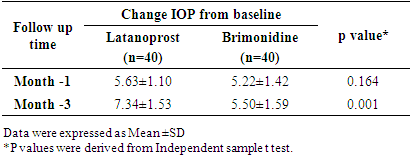
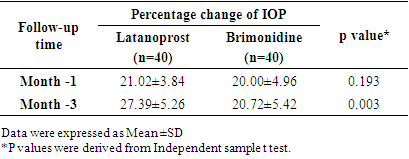
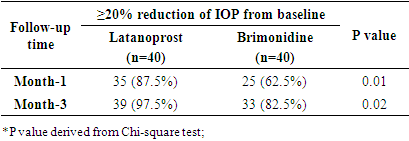
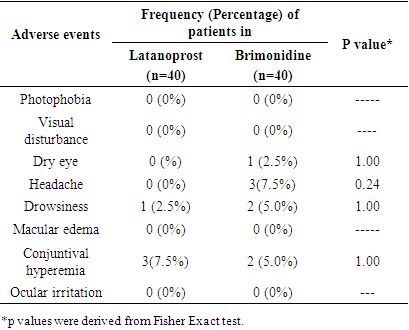
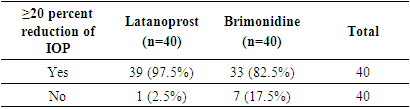
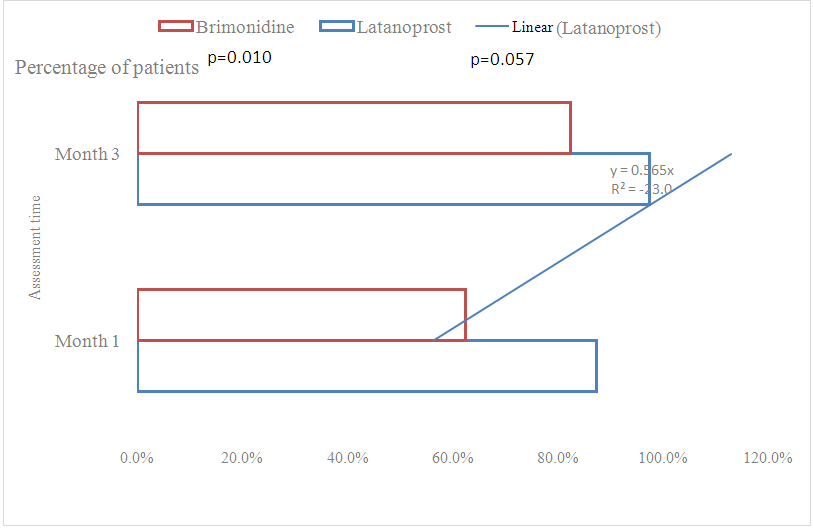
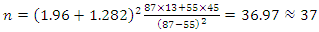 Adjusted for 5% patients lost to follow-up:nc =n +n×l = 37+37×0.05 ≈ 40This means that a sample size of 40 subjects per arm was needed to test the hypothesis.
Adjusted for 5% patients lost to follow-up:nc =n +n×l = 37+37×0.05 ≈ 40This means that a sample size of 40 subjects per arm was needed to test the hypothesis. 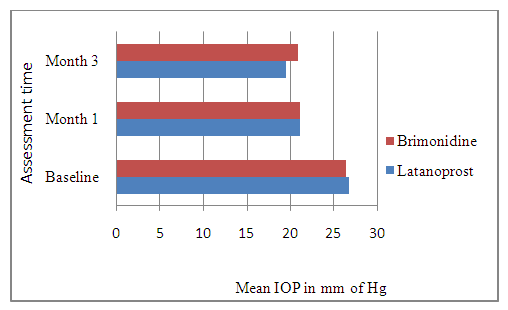
 Particulars of the patient:Name:Age:
Particulars of the patient:Name:Age:  Sex: Male/Female. Marital status: M/Um. Iris color: Blue/Brown/Green/Hazel Add:Guardian’s name:
Sex: Male/Female. Marital status: M/Um. Iris color: Blue/Brown/Green/Hazel Add:Guardian’s name:  Drug history:Chief Complaints: H/O past illness: CVD/HTN/DM/BA. F/H: DM/HTN/BAGeneral exam:
Drug history:Chief Complaints: H/O past illness: CVD/HTN/DM/BA. F/H: DM/HTN/BAGeneral exam:  Pulse b/m BP:
Pulse b/m BP: mm of HgDiagnosis: POAG (Bilateral). Follow Up Chart:
mm of HgDiagnosis: POAG (Bilateral). Follow Up Chart: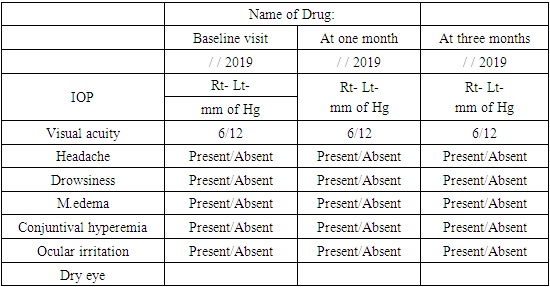
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML