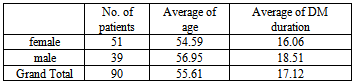Mohamed Yasser Sayed Saif 1, Ahmed Tamer Sayed Saif 2, Passant Sayed Saif 3, Hany Salah al-Din Saftawy 4
1Department of Ophthalmology, Beni Sueif University, Egypt
2Department of Ophthalmology, Beni Sueif University, Fayoum University, Al Fayoum
3Department of Ophthalmology, Beni Sueif University, Misr University for Science and Technology, Cairo, Egypt
4Department of Ophthalmology, Research Institute of Ophthalmology
Correspondence to: Mohamed Yasser Sayed Saif , Department of Ophthalmology, Beni Sueif University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Aims: this study assesses the effectiveness of anti-VEGF therapy for preserving or improving vision in people with diabetic macular oedema (DME). Settings and Design: none randomized controlled trial. It was carried out between March, 2009–2011. Material and Methods: intravitreal Bevacizumab injection 0.05ml / 1.25mg on 90 patients with 106 eyes with diabetic macular oedema. Results: the age ranged from 40 to 85 years with a mean age of 55.61 (54.59 for females and 56.95 for males). Patient received from 1–2 injections of Bevacizumab with a mean of 1.04 + 0.19 according to the FFA and OCT follow up. Eighteen eyes (17%) received the intravitreal injection alone, 31 eyes (29%) had macular grid in conjunction with the intravitreal injection and the rest 57 eyes (54%) had PRP with the injection according to the presence of associated nonproliferative of proliferative changes in the fundus. Conclusions: anti-VEGF therapy is effective in the treatment of DME. Several short-term prospective studies have shown visual improvement and reduced retinal thickness with treatment. DME not as responsive as PDR and further long-term study is needed.
Keywords:
Anti-VEGF Therapy, Diabetic Macular Oedema, Intravitreal Bevacizumab
Cite this paper: Mohamed Yasser Sayed Saif , Ahmed Tamer Sayed Saif , Passant Sayed Saif , Hany Salah al-Din Saftawy , Intravitreal Bevacizumab in Diabetic Macular Oedema, Research in Ophthalmology, Vol. 2 No. 2, 2013, pp. 15-20. doi: 10.5923/j.ophthal.20130202.01.
1. Introduction
Diabetic retinopathy is the leading cause of blindness in people of working age. Diabetic macular oedema (DME) affects approximately 29% of diabetic patients with disease duration of 20 years or more and is the main reason for reduced vision in this segment of population. [1]The first line of treatment remains the management of systemic risk factors but is often insufficient in controlling DME and, laser retinal photocoagulation is considered the standard of care. However, laser treatment reduces the risk of moderate visual loss by approximately 50% without guaranteeing remarkable effects on visual improvement. [2]However, there have been patients with diffuse diabetic macular edema refractory to such treatment modalities. This is because of the structural damage caused by chronic macula edema and the underlying diabetic retinopathy. Hence, antiangiogenic therapy with anti-vascular endothelial growth factor (anti-VEGF) modalities has been proposed for improving vision in people with DME. [3]AimThis study assesses the effectiveness of anti-VEGF therapy for preserving or improving vision in people with DME.
2. Materials and Methods
This is a non randomized uncontrolled study. The study was carried out between March, 2009–2011 on 90 patients with 106 eyes with diabetic macular oedema (focal, diffuse, and non-ischemic). Both genders were eligible for study with an OCT thickness in the center of the macula ≥ 275µm.Full ophthalmic examination including diabetic history, previous injections or treatments, visual acuity, IOP examination, slit lamp examination including fundus biomicroscopy (contact and noncontact), indirect ophthalmoscopy, Fluorescein Fundus Angiography (FFA), and Optical Coherence Topography (OCT) was done to all patients in the study.All patients were checked and controlled for diabetes, blood pressure, and lipid profile by their internal physician prior to injection.All patients were prepared for intravitreal injections. All patients received topical and systemic antibiotics (Gatifloxacin eye drops, Cephadroxil Monohydrate 500mg /cap)Operative procedure• Topical 0.4 % Benoxinate HCL eye drops were applied.• Ocular sterilization and draping.• Application of eye speculum.• Measuring the site of injection using a caliber at a site of 4mm from the limbus in phakic eyes and 3.5 mm in pseudophakic eyes.• The needle is pointed towards the mid-vitreous cavity and perpendicular to the globe.• Intravitreal injection of 0.05 ml of Bevacizumab (1.25mg).• Securing the removal of the syringe by a tip of cotton swab to prevent reflux of Bevacizumab or vitreous from the injection site.• Application of combined antibiotic and steroid eye ointement (tobramycin and dexamethazone).• Removal of eye speculum.• Eye patching by sterile bandage.All patients were followed up in the first day, 1 week , 2 week, 1 month, 3 month, and 6 month in which full ophthalmic examination was done in all visits but FFA and OCT was done preinjection and after 6 month for all.Patients had macular grid or PRP treatment according to the presence of associated nonproliferative of proliferative changes in the fundus together with the intravitreal injections.
3. Results
Ninty patients with 106 eyes were included in this study. Age ranged from 40 to 85 years with a mean age of 55.61 (54.59 for females and 56.95 for males). All patients were diabetics from 10–35 years (mean 17.12 + 5.5). The demographic data for the patients in the study are shown in Table 1 including the age and duration of diabetes mellitus.Table 1. Demographic data
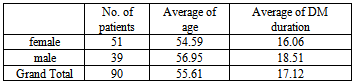 |
| |
|
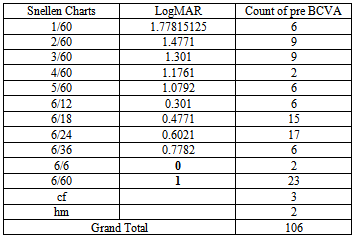
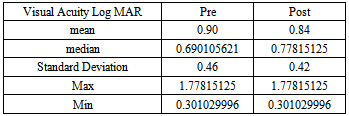
The visual acuity was recorded using the Landolt’s broken rings before and after Bevacizumab injection and converted to LogMAR for statistical analysis. There were 69 eyes improved in VA, 30 eyes stable and 7 eyes worsen in VA as shown in Tables 2, 3a, and 3b and in Figure 1.Table 2. Best corrected visual acuity pre injection
 |
| |
|
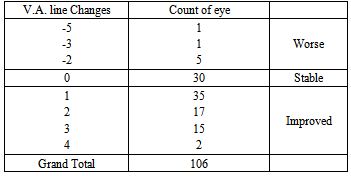
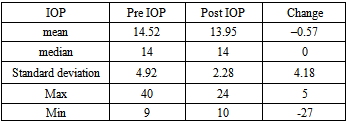
The preinjection IOP ranged from 9–40mmHg with a mean of 14.52+4.92mmHg, while the postinjection IOP ranged from 10–24 mmHg with a mean of 13.95 +2.28 mmHg. The mean decrease of IOP was 0.57 ± 4.18 mmHg as shown in Table 4.Table 3a. The number of lines changed from pre operative visual acuity for all patients in the study
 |
| |
|
Table 3b. The data analysis of pre and post injection visual acuity in LogMAR. There are five patients with a previsual acuity of CF and HM cannot be converted to LogMAR
 |
| |
|
Table 4. Statistical analysis of IOP changes during the study
 |
| |
|
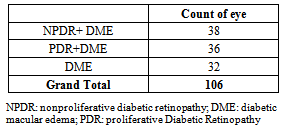
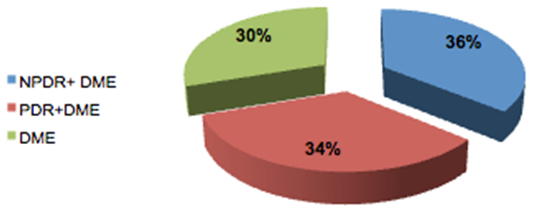
Elevation of intraocular pressure was reported in 33 eyes (31.1%) while 30.1% remained stable and 38.6% decreased.The fundus examination of the patients showed that 32 eyes had DME alone while 38 eyes had nonproliferative diabetic retinopathy (NPDR) together with DME and 36 eyes had proliferative diabetic retinopathy (PDR) together with DME as shown in Table 5 and Figure 2.Table 5. Showed the fundus findings associated with the DME
 |
| |
|
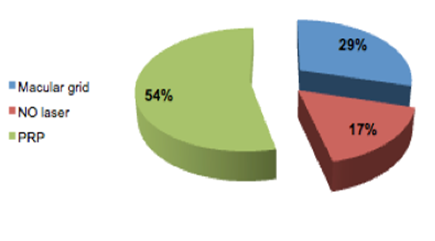
In this study, patient received from 1–2 injection of Bevacizumab with a mean of 1.04+0.19 according to the FFA and OCT follow up (only four eyes received two injections).Eighteen eyes (17%) received the intravitreal injection alone, 31 eyes (29%) had macular grid in conjunction with the intravitreal injection and the rest 57 eyes (54%) had PRP with the injection [Figure 3].Pre- and post Injection in a 74-year-old female patient with DME are demonstrated in Figures 4 and 5.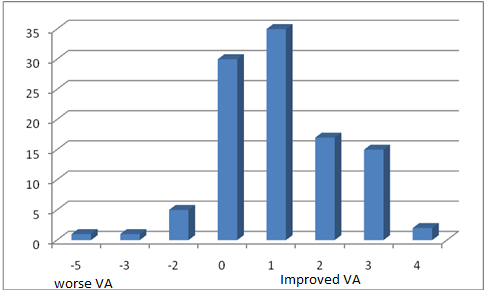 | Figure 1. The number of lines changed from preoperative visual acuity for all patients in the study |
 | Figure 2. Showed the fundus findings associated with the DME |
 | Figure 3. Management performed to the diabetic macular edema patients (PRP: pan retinal photocoagulation) |
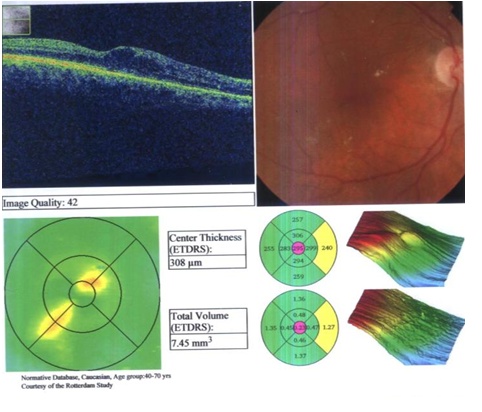 | Figure 4. Preinjection in a 74-year-old female patient with DME with a central thickness of 308um |
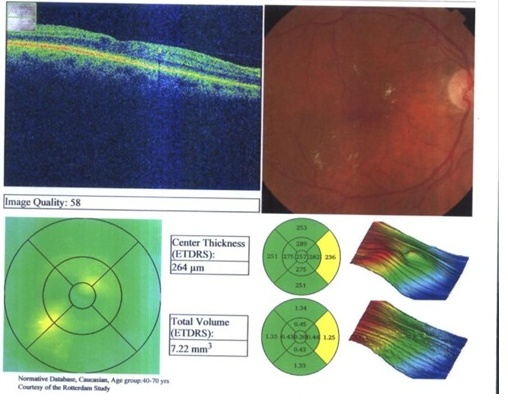 | Figure 5. Postinjection after 8 weeks in the same 74 Old female patient with DME with a central thickness decreased to 264um |
4. Discussion
Diabetic macular edema is the leading cause of decreased vision from diabetic retinopathy. This decreased vision is caused by an increase in extracellular fluid within the retina distorting the retinal architecture and frequently taking on a pattern of cystoid macular edema. This fluid accumulates within the retina because of the breakdown of the barriers within the retinal blood vessels and possibly the pigment epithelium. Diabetic macular edema tends to be a chronic disorder. Although spontaneous recovery is not an uncommon occurrence, over one-half of diabetics with macular edema will lose two or more lines of visual acuity within 2 years. [4]The ETDRS showed that laser photocoagulation stabilized vision and reduced the risk of visual loss by 50%. [5] Laser photocoagulation and IVTA results were not satisfactory in many cases because of recurrent or persistent diabetic macular edema. The vitreous has been implicated as a cause of diabetic macular edema via several mechanical and physiologic mechanisms, all of which led to increased vascular permeability. [6-11] In our study the intravitreal injection of avastin was enough in 17% and in with macular grid 29% and PRP in 54% of eyes. Suggested mechanisms include destabilization of the vitreous by abnormal glycation and crosslinking of vitreal collagen leading to traction on the macula, accumulation and concentration of factors causing vasopermeability in the premacular vitreous gel, and accumulation of chemoattractant factors in the vitreous, leading to migration of cells to the posterior hyaloid, contraction, and macular traction. [6-11] Various studies suggest that the presence of tangential vitreomacular tractional forces combined with the local presence of a number of cytokines (e.g. interleukin-6) and growth factors (vascular endothelial growth factor and angiotensin) contribute to the development of diabetic macular edema, and their removal benefits macular edema and helps to delay its progression. [12]It was demonstrated that retinal hypoxia plays a role in DME and VEGF, which is upregulated by hypoxia, and is likely to contribute to the excessive vascular permeability that results in macular edema in people with diabetes. Several studies have demonstrated not only a correlation of VEGF levels with the severity of diabetic retinopathy, but also a reduction in levels after successful laser treatment of PDR. [13,14] Thus, a rational approach to treating macular edema in these patients would include the use of anti-VEGF agents. [15]Progression of PDR was distinguished by a sustained, upregulated expression of VEGF by the neurosensory retina. Cells in all retina layers can potentially contribute to augmented VEGF production. The restricted population of VEGF producing cells in each case is likely to represent cells residing in ischaemic regions of the retina. Thus, VEGF function as a linking factor between retinal ischaemia and PDR-associated neovascularization. [16]The results of a clinical trial show an increase in 65% in BCVA at all postoperative study visits when compared with baseline values. These visual results were better than expected from the photoreceptor damage caused by chronic macular edema, underlying diabetic retinopathy changes, and prior macular laser photocoagulation treatment which was an inclusion criterion for the study. However, this marked visual improvement might also reflect the cases of diabetic macular edema that were excluded from the trial, such as those with ischemic maculopathy, neurosensory detachment, and active proliferative diabetic retinopathy, which are the most common causes of lack of visual improvement in diabetic macular edema. But 28% of patients showed no improvement in BCVA while 6.6% got worse. In comparison Arevalo et al. demonstrated that the final BCVA analyses by subgroups were improved two or more ETDRS lines of BCVA in 5 (11.6%) eyes, while 35 (81.4%) eyes remained stable, and 3 (7%) eyes decreased two or more ETDRS lines of BCVA. [15]The limited functional results in this study may have been attributable to the included cases of proliferative diabetic retinopathy (36 patients). Although there have been a considerable number of studies indicating an important role of the posterior hyaloid in the pathogenesis of diabetic macular edema, we did not find any additional effect of posterior hyaloid detachment over macular grid laser in this study. One possible cause for this discrepancy is the chronic nature of diabetic macular edema and longstanding intraretinal diabetic changes with irreversible photoreceptor damage, and the selection of patients with type-2 diabetic mellitus, which may have had a negative impact on photoreceptor recovery in this elderly study population. The patients selected represent the typical situation in most elderly patients with type-2 diabetes where no clinically visible vitreomacular traction is present.In this trial, there was a highly statistically significant decrease in central foveal thickness at all postoperative study visits when compared with baseline values in all cases.It was also observed that the mean central foveal thickness showed maximum improvement at 3 months, with a subsequent small gradual increase in central foveal thickness at 6 and 12 months to patients who did not receive macular grid or panretinal photocoagulation either prior or 2 weeks after injection; however, this was still markedly less than baseline values. This is because the effect of the injected drug lasts for a short duration and the requirement for multiple reinjections are the major drawback of the presently available drugs. Also, further deterioration is very likely if macular edema redevelops. [17-19].In our study only four eyes that need multiple injections while the rest (102 eyes) received only one injection. This shows the importance of double therapy of Bevacizumab together with grid and PRP for long-term treatment of Diabetic macular edema.With regard to IOP there was decrease in the mean IOP of 0.57 + 4.18 mmHg from the baseline. In our study, there was elevation of intraocular pressure in 33 eyes (31.1%), while 30.1% remained stable and 38.6% decreased than the baseline. This is comparable to Kim et al. who evaluated the long-term intraocular pressure (IOP) changes after intravitreal injection of bevacizumab for age-related macular degeneration. A total of 83 eyes that received intravitreal injections of bevacizumab for age-related macular degeneration were enrolled. There was no significantly higher elevation than baseline IOP (14.11 ± 2.76 mmHg) after multiple intravitreal injections of bevacizumab (P>0.05). In the patients with pre-existing glaucoma (three eyes), there were no significant increases of IOP during the follow-up period. [20]IOP changes were not compararple to Flakenstein et al. [21] who measured the changes of intraocular pressure after intravitreal injection of bevacizumab (avastin) in 70 patients (122 injections) for exudative age-related macular degeneration treatment. Forty-one eyes (59%) had single injection, 29 eyes (41%) had repeated injections. IOP was measured before and after Avastin injection at 3, 10, and 15 min. Avastin injections caused a predictable probably volume-related rise in IOP that never occluded the central retinal artery and which spontaneously fell to below 30 mmHg in all eyes within 15 min.
5. Conclusions
Anti-VEGF therapy holds promise in the treatment of DME. Several short-term prospective studies have shown visual improvement and reduced retinal thickness with treatment. DME not as responsive as PDR and further long-term study is needed.
References
| [1] | Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984;91:1464-74. |
| [2] | Bandello F, Berchicci L, La Spina C, Battaglia Parodi M, Iacono P. Evidence for anti-VEGF treatment of diabetic macular edema. Ophthalmic Res 2012;48 Suppl 1:16-20. |
| [3] | Antonopoulos C, Subramanian M. Diabetic Macular Edema, Diabetic Retinopathy, Dr. Mohammad Shamsul Ola, editor. Chap. 10 2012. p. 193-206 ISBN: 978-953-51-0044-7, InTech, Available from:http://www.intechopen.com/books/diabetic-retinopathy/diabetic-macular-edema [Last accessed on Feb 2013 |
| [4] | Ferris FL 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 1984;28 Suppl: 452-61. |
| [5] | The Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report No 1. Arch Ophthalmol 1985;103:1796-806. |
| [6] | Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina 2000;20:126-33. |
| [7] | Harbour JW, Smiddy WE, Flynn HW, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol 1996;121:405-13. |
| [8] | Heij EC, Hendrikse F, Kessels AG, Derhaag PJ. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol 2001;239:264-70. |
| [9] | Dillinger P, Mester U. Vitrectomy with removal of the internal limiting membrane in chronic diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol 2004;242:630-7. |
| [10] | Figueroa MS, Contreras I, Noval S. Surgical and anatomical outcomes of pars plana vitrectomy of diffuse nontractional diabetic macular edema. Retina 2008;28:420-6. |
| [11] | Kuhn F, Kiss G, Mester V, Szijártó Z, Kovács B. Vitrectomy with internal limiting membrane removal for clinically significant macular oedema. Graefes Arch Clin Exp Ophthalmol 2004;242:402-8. |
| [12] | Saeed AM. Combined vitrectomy and intravitreal injection versus combined laser and injection for treatment of intractable diffuse diabetic macular edema. Clin Ophthalmol 2013;7:283-97. |
| [13] | Tolentino MJ, McLeod DS, Taomoto M, Otsuji T, Adamis AP, Lutty GA. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol 2002;133:373-85. |
| [14] | Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. m J Ophthalmol 2005; 139:476-81. |
| [15] | Arevalo JF, Sanchez JG, Lasave AF, Wu L, Maia M, Bonafonte S, t al. Intravitreal Bevacizumab (Avastin) for diabetic retinopathy: The 2010 GLADAOF Lecture. J Ophthalmol 011;2011:584238. |
| [16] | Pe'er J, olberg R, Itin A, Gnessin H, emo I, eshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. r J Ophthalmol 1996;80:241-5. |
| [17] | Jonas JB, Spandau UH, Kamppeter BA, Vossmerbaeumer U, Harder B. Follow-up after intravitreal triamcinolone acetonide for diabetic macular edema. Eur J Ophthalmol 006;16:566-72. |
| [18] | Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: Two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology 2006;113:1533-8. |
| [19] | Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006;26:999-1005. |
| [20] | Kim D, Nam WH, Kim HK, Yi K. Does intravitreal injections of bevacizumab for age-related macular degeneration affect long-term intraocular pressure? J Glaucoma 2013 Apr 29. [Epub ahead of print] |
| [21] | Falkenstein IA, Cheng L, Freeman WR Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina 2007;27:1044-7. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML