-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Nursing Science
p-ISSN: 2167-7441 e-ISSN: 2167-745X
2019; 9(2): 25-29
doi:10.5923/j.nursing.20190902.01

Effect of Apus Bamboo Shoot Extract on Total Interleukin 17 and Leukocyte at Atherosclerosis Process
Edy Soesanto1, Edi Dharmana2, Soeharyo Hadisaputro2, S. Fatimah Muis2
1Department of Nursing, University of Muhammadiyah Semarang, Indonesia
2The Great Teachers Faculty of Medicine Diponegoro University of Semarang, Indonesia
Correspondence to: Edy Soesanto, Department of Nursing, University of Muhammadiyah Semarang, Indonesia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Atherosclerosis is a consequence of inflammation and excess of oxidative stress. High level of IL-17 and leucocytes are considered as the risk of rupture of plaque, thrombus, and embolism due to the acceleration of blood vessels lession and blockage Bamboo shoots apus possesses antioxidant content and it possibly inhibits the atheroslerosis progress. The purpose of this study was to prove the influence of bamboo shoot extract to decrease interleukin 17 and leucocyte count in New Zealand White rabbit with atherogenic diet. This research used 1-2 weeks freeze dried apus bamboo shoot extract and administered in New Zealand White rabbit. The atherogenic diet consisted of standard feed added by 0.5% egg yolk and 5% pork oil. This research used Randomized pre and post-test with control group design by dividing (jumlah kelinci) rabbits into 4 groups. The results showed that there was a significant difference of IL-17 levels between treatments and all groups had decreased levels of IL-17 except in the positive control group that increased by 57.1% and the highest decrease occurred in treatment group 3 which was 65.4%. There was a significant difference in the number of absolute leukocytes between treatments (p = 0.001). The increase of absolute leukocyte count of positive control group with treatment group 1, 2 and 3 was significantly different (p = 0.0001). In conclusion, bamboo shoot extract is able to decrease interleukin 17 level and absolute leukocyte count.
Keywords: Bamboo shoot, Bamboo shoot extract, IL-17, Leukocytes
Cite this paper: Edy Soesanto, Edi Dharmana, Soeharyo Hadisaputro, S. Fatimah Muis, Effect of Apus Bamboo Shoot Extract on Total Interleukin 17 and Leukocyte at Atherosclerosis Process, International Journal of Nursing Science, Vol. 9 No. 2, 2019, pp. 25-29. doi: 10.5923/j.nursing.20190902.01.
Article Outline
1. Preliminary
- Atherosclerosis is the most common cause of coronary artery disease, carotid artery disease, peripheral artery disease and is the global major cause of death. Atherosclerosis is a progressive disease, progressing slowly but surely and increasing by 3 percent annually in the age of 20 (Nurahmi, et.al 2016). Heart disease and blood vessels, which lead to atherosclerosis as the cause of death in Indonesia, raised from 5.1% to 8.7% in the age range 45-54 years and the increased incidence of atherosclerosis in patients aged more than 40 years is 17-40% (Indonesia DKR, 2010).Atherosclerosis is the consequence from multiple factors, including inflammatory processes and oxidative stress. Endothelial dysfunction, caused by ox-LDL, and inflammatory processes, resulting in activation of monocyte migration into the intima, leading to atherosclerosis (Li D, 2005). The occurrence of inflammatory processes in atherosclerosis is related to the role of cytokines and leukocytes. High levels of IL-17 in the vascular worsen the stability of atherosclerotic plaques bylesions formation. These lessions in turn promote plaque rupture (Hashmi, 2006). One of the indicators in initiation and the onset of integrated atherosclerosis from acute and chronic inflammatory responses is an increase of leukocytes number (Lee D.C et.al. 2001). An elevated circulating leukocytes may increase blood vessel blockage and increase risk of plaque rupture which result in thrombus and emboli ending with myocardial necrosis (Homenta et al. 2009).In the world of health is currently being intensified the concept of "food as medicine" and well known as functional food which overcome the disease. Functional food ingredients are expected to inhibit the progression of atherosclerosis due to its antioxidant content. Several ingredients which contribute as an inflammatory process in atherosclerosis, which is a vitamin E, pholipenol, plavonoid, vitexin and orientin, palmitic acid, curcumene, limonene, toluene, naphthalene, 1,3,5-trimethyl benzene, (Lu B. Et.al. 2010). Other antioxidant compounds present in bamboo are vitamin A, thiamin, riboflavin, vitamin C, curcumin (Choudhury D, 2010). The purpose of this study was to prove the influence of bamboo shoot extract to decrease interleukin 17 and leucocyte count in New Zealand White rabbit with atherogenic diet.
2. Materials and Methods
- The bamboo shoot aged 1-2 weeks was gained from Banyumeneng village, Mranggen district. The bamboo shoot was extracted by freeze dried method using ethanol 90% (Soesanto E, 2016). The standard feed for rabbits used was Feed Premium rabbit produced byPT Cargill Indonesiaand has nutritional content per 100 gr: 5% fat, 18% protein, crude fiber 14%, 1% calcium, 0.8% phosphorus, ash 12% and water 12%. The diet was given maximum 5% of the rabbit's weight and beverages weregiven maximum 10% of the rabbit's weight.The atherogenic diet used was standard diet added by 0.5% egg yolk and 5% pork oil (Soesanto, E. 2017). Cholesterol flour is made from egg yolks dried oven at 60 °C for 24 hours to dry and blend until smooth. The experimental animal used was 4-month-old New Zealand White rabbit weighed between 2000-2500 grams, and male sex. Standard diet as well as atherogenic dietwere provided as much as 100 grams / day / tail and ad libitum water. Absolute leukocyte examinedfrom whole blood plus EDTA and analyzed by Hematology Analyzer while IL-17 was assessed using quantitative sandwich enzyme immunoassay technique which was conducted at Integrated Research and Testing Laboratory (LPPT) Gajah Mada University Yogyakarta. This research used pure experimental, Randomized pre and post-test design with control group design which have got approval from Health Research Ethics Commission FK UNDIP and Dr. Kariadi Semarang no. 23 / EC / H / FK-RSDK / 2017 dated May 22, 2017. This study used 24 rabbits which were divided into 4 groups ie control group:the group given Atherogenik diet, treatment group I, II and III respectively were given Atherogenik diet plus bamboo shoot extract with concentration 130 mg / kg Weight, 260 mg / kg Weight, and 520 mg / kg Body Weight.
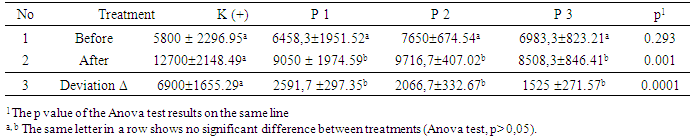
3. Result and Discussion
- The mean of IL-17 level of all samples before treatment was 281.7 ± 130.14 pg / mL with a range of 124.8-570.5 pg / mL, while the mean of IL-17 concentration of all samples after treatment was 166.3 ± 98.53 pg / mL with a hose between 51.2-496,7 pg / mL.
|
|
4. Conclusions
- The bamboo shoot extract is found to have a beneficial effects on the decrease of IL-17 and absolute leukocyte count in New Zealand White rabbit given atherogenic diet. The dose 520 mg / kg / day is an effective dose to decrease IL-17 and leukocytes level.
Suggestion
- Further research is needed to examine the toxicity before continuing the clinical trial to human. Furthermore, apus bamboo shoot extract can be one of the standardized ingredient alternatives of traditional medicine.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML