-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Nursing Science
p-ISSN: 2167-7441 e-ISSN: 2167-745X
2016; 6(3): 67-72
doi:10.5923/j.nursing.20160603.01

The Effect of Obesity on Ovulation; A Prospective Study on Females Seeking Fertility in Beni-Suef, Egypt
Hanaa Kamal Helmy1, Amel Abd Elazim Mohamed2, Momen Zakaria Mohamed3, Ahmed Emad El Din Arafa4
1Lecturer of Maternal and Newborn Health Nursing, Faculty of Nursing, Beni - Suef University, Egypt
2Lecturer of Community Health Nursing, Faculty of Nursing, Beni-Suef University, Egypt
3Department of Obstetrics and Gynecology, Faculty of Medicine, Beni-Suef University, Egypt
4Department of Public Health, Faculty of Medicine, Beni-Suef University, Egypt
Correspondence to: Hanaa Kamal Helmy, Lecturer of Maternal and Newborn Health Nursing, Faculty of Nursing, Beni - Suef University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

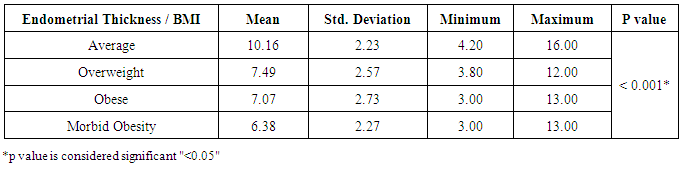
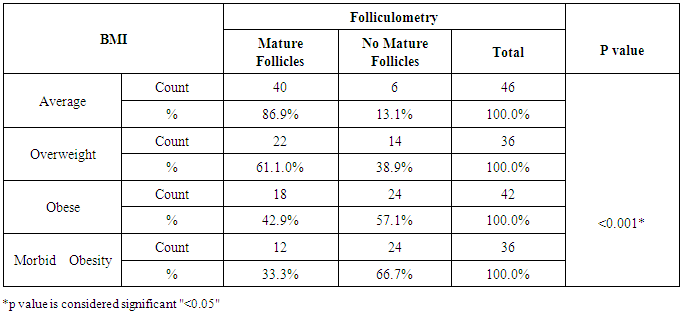
Background: Obesity has been associated with impaired fertility, furthermore obese women has experience of disturbance in the menstrual cycle, hypothalamic-pituitary ovarian axis and unfortunately a very high prevalence of infertility among obese population The aim of the study is to detect the effect of obesity on oocyte quality, ovulatory hormones and endometrial pattern in females seeking fertility in Beni-Suef, Egypt. SubjectsandMethods: The study is a prospective comparative study that was carried out on 160 participants, seeking fertility. Patients were selected randomly from infertile women attending Beni-Suef General Hospital outpatient obstetrics and gynecology clinics. All subjects were prescribed to clomiphene citrate. Patients were divided according to their BMI to average weight, overweight, obese and morbidly obese groups. Hormonal profile and investigational radiations were done to assess patients' ovulation, oocyte quality, ovulatory hormones and endometrial pattern. Results: Subjects with morbid obesity had significantly higher levels of FSH and prolactin, and lower levels of day 21 progesterone compared to other groups (p<0.05). Also, subjects with average weight had significantly thicker endometrium compared to other groups (p<0.05) and the lower the BMI the higher the U/S evidence of mature follicles (p<0.05). Conclusions:Obesity is suggested to have a negative impact on the dynamics of ovulatory hormones, quality of oocyte and thickness of endometrium; and therefore may reduce fertility.
Keywords: Obesity, Ovulation, Infertility, Fertility
Cite this paper: Hanaa Kamal Helmy, Amel Abd Elazim Mohamed, Momen Zakaria Mohamed, Ahmed Emad El Din Arafa, The Effect of Obesity on Ovulation; A Prospective Study on Females Seeking Fertility in Beni-Suef, Egypt, International Journal of Nursing Science, Vol. 6 No. 3, 2016, pp. 67-72. doi: 10.5923/j.nursing.20160603.01.
Article Outline
1. Introduction
- The obesity was defined by World Health Organization as body mass index (BMI) ≥30 kg/m. However the obesity is associated with comorbid condition such as heart disease, diabetes mellitus, malignancies and fertility problems [1].Unfortunately, the obesity is an increasingly prevalent health burden upon modern society. The World Health Organization (WHO) estimates that 1.6 billion people worldwide are overweight and 400 million are obese [2]. Rates of obesity in the developing world have tripled in the last two decades [3]. In the USA and UK more than half of all women are either overweight or obese [4], and many are at reproductive age [5].Most obese women are not infertile; however, obesity and its negative impact upon fecundity and fertility are well documented. Furthermore, the obese woman are three times more likely to suffer infertility than women with a normal body mass index (BMI) [6]. However Obese women experience impaired fecundity both in natural and assisted conception cycles. A Dutch study monitored the effect of obesity upon fecundity in a donor insemination program; 500 women were prospectively monitored. The researchers concluded that the increase in weight led to a decrease in probability of conception per cycle [7]. Furthermore, Obesity can exert effects upon the hypothalamic– pituitary–ovarian (HPO) axis and as such disturb menstrual cycle and ovulation. A large questionnaire study of 26 638 women demonstrated that menstrual cycle irregularity and anovulation were correlated with being overweight or obese. Indeed, the grossly obese women had a rate of menstrual disturbance 3.1 times that of women with normal weight [8]. In addition; the health risk associated with obesity, the deterioration effects on reproductive function and pregnancy outcomes. Studies have reported that women with obesity have decreased pregnancy and increased miscarriage rate [9].Whilst several studies have proposed disturbance to the HPO axis as a key pathophysiological factor in sub-fecundity in the obese women, impaired fertility has been demonstrated in obese women with normal menstrual cycle. A retrospective analysis of US data collected as part of the Collaborative Perinatal Project demonstrated a reduced fecundity for overweight and obese women. Fecundity remained reduced in overweight and obese women when only women with normal menstrual cycles were considered [10].Besides, it is established that a complex hormonal orchestra works in balance to control the menstrual cycle, ovulation and development of the endometrium. Obesity has been demonstrated to perturb this balance via several direct and indirect mechanisms. Adipose tissue has been shown to disturb sex hormone secretion and bioavailability. Indirectly, obesity exerts its effect via leptin, insulin and the adipokines [4, 5].The state of insulin resistance with secondary hyperinsulinaemia is commonly observed in obese, infertile women. Gonadotrophic effects of insulin on ovarian steroid hormone synthesis were shown in vitro and in vivo. The exaggerated insulin action on the ovarian tissue may present the pathogenic mechanism leading to the disturbances of the endocrine profile and menstrual cycle, and hence to infertility in some obese women [11].Moreover, the effect of obesity upon implantation rate has been inconsistently reported. Some authors have identified a reduction in implantation rates among the obese women [12, 13]. An unfavorable intrauterine milieu and impaired endometrial receptivity are plausible loci for the effect of obesity upon sub-fecundity. Also, obese women tend to suffer non-recurrent spontaneous pregnancy loss [13, 14]. This suggests that both the endometrium and oocyte quality are likely to play pivotal roles.Based on the cited facts, this study aims at assessing the effect of obesity on oocyte quality, ovulatory hormones and endometrial pattern in females seeking fertility in Beni-Suef, Egypt. The study introduces answers to a main general question about the possible impact of obesity on ovulation and fertilization and three secondary questions about the effect of obesity on each of oocyte quality, hormonal profile and endometrial pattern.Significance of the studyThe significance of the study spins around the undermining effect of obesity on ovulation and infertility which turns the light towards the pivotal role nurses and nutritionists have to play in commencing weight reduction programs to infertile women based on healthy diets of lower calories and sport practice in order to enhance women ovulation.
2. Subjects and Methods
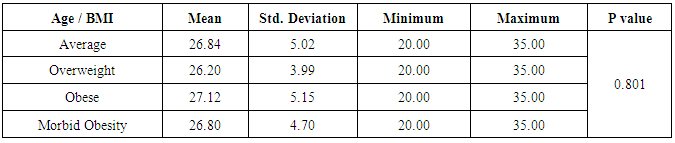
- The aim of the study was to assess the effect of obesity on oocyte quality, ovulatory hormones and endometrial pattern in females seeking fertility in Beni-Suef, Egypt.Research design The study was a prospective comparative study that was carried out on 160 participants, seeking fertility, subjected to induction of ovulation, and underwent vaginal U/S and basal hormonal profile labs to assess their ovulation status in Beni-Suef General Hospital.Study SettingThe study was Conducted at Beni-Suef General Hospital Obstetrics and Gynecology outpatient clinics.Hypothesis:We hypothesize that there obesity may have an impact on ovulation and different stages of obesity may have varying effects on ovulation. Simply, the study shows the impact of different grades of obesity on ovulation, oocyte quality, hormonal profile, radiations and endometrial pattern to detect whether obesity has a negative or no impact on fertility.Subjects:Participants were divided according to their BMI to 4 groups:• Group 1: Average weight (BMI 18-24.9 kg/m²); 46 subjects.• Group 2: Overweight (BMI 25-29.9 kg/m²); 36 subjects.• Group 3: Obese (BMI 30-34.9 kg/m²); 42 subjects.• Group 4: Morbid obesity (BMI >35 kg/m²); 36 subjects.Inclusion criteria:1. Age: between 20 and 35 years.2. No history of medical diseases that may affect ovulation.Exclusion criteria:1. Age: < 20 years or > 35 years.2. Lactating women. 3. Women with amenorrhea. 3. History of medical diseases that may affect ovulation. 4. Patients who had previous operations in the ovary such as ovarian cystectomy.Methods:First all subjects were informed of all details of the study including its purposes and expected benefits and outcomes.Only patients who signed informed consents were subjected to the following:1. History: detailed history taking; including personal history, socio-demographics, medical and obstetric history, history of medications, family history and history of operations using a simple questionnaire to detect whether the subjects were oblige to the study or not.2. Physical examination: general and local classic physical examinations were done.3. Anthropometric measurements: weight, height, waist circumference, triceps skin fold thickness in order to put the patients into their appropriate categories.4. Laboratory investigations: labs included FSH, LH, Prolactin and day 21 progesterone in order to investigate the possible effects of obesity on the hormonal profile and consequently ovulation.5. Radiological investigations: all participants were given induction of ovulation. Then, ovulation was monitored by vaginal U/S folliculometry; starting day 9 or 10 of last menstrual period then day after day. Mature follicles were considered follicles >18mm by U/S and endometrial pattern by vaginal U/S. All radiations were done by the same operator and the same U/S machine to avoid inter and intra observer "operator" bias, and also to avoid machinery bias.Field workThe researcher attended Beni-Suef General Hospital Obstetrics and Gynecology outpatient clinics in the period between August 2015 and February 2016. Patients were selected randomly according to a classic simple random sampling.Ethical considerations:The study was approved by the ethical committee of the Faculty of Medicine and Nursing Beni- Suef University. The subjects were informed of the purpose of the study and its consequences with confirming confidentiality of data.Statistical analysis:Data were analyzed using the software, Statistical Package for Social Science, (SPSS) version 20, then processed and tabulated. Frequency distribution with its percentage and descriptive statistics with mean and standard deviation were calculated. Chi-square, t-test, correlations were done whenever needed. P values of less than 0.05 were considered significant.
3. Results
|
|
|
|
|
|
|
4. Discussion
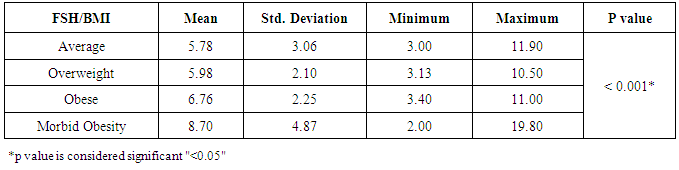
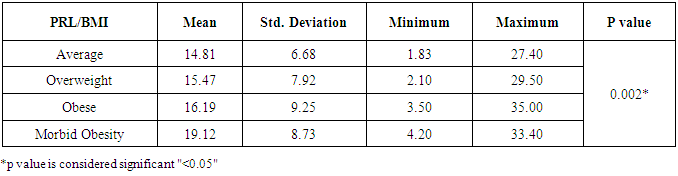
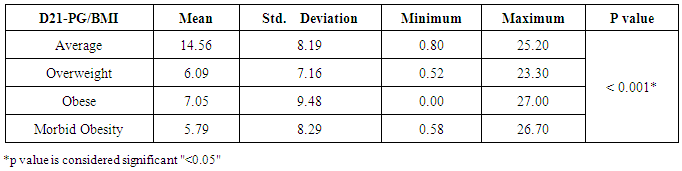
- Obesity and overweight are common conditions in the developed countries and they carry many health consequences, including some reproductive disorders. There is a very high prevalence of obese women in the infertile population and many studies have highlighted the link between obesity and infertility [2-7].In our study, there was no statistically significant difference between the groups regarding their LH levels; average weight 7.15±3.81 IU/L, overweight 7.75±4.02 IU/L, obese 6.55±2.78 IU/L and morbid obesity 9.05±4.15 IU/L (p>0.05). The study also showed that women with morbid obesity had significantly higher levels of FSH compared to other groups; average weight 5.78±3.06 IU/L, overweight 5.98±2.10 IU/L, obese 6.76±2.25 IU/L and morbid obesity 8.70±4.87 IU/L (p<0.05). Our results consist with Sathya et al., who studied relation between BMI and menstrual cycles and stated in their results that the level of basal LH did not differ between obese and lean women [15]. Also Giovanni et al. found lower basal FSH and estradiol levels in obese patients in their study that was carried on 22 patients with BMI >30 to document the hormonal differences in obese women [16]. However, our finding contradicts a previous study by Butzow et al. which showed increased secretion of LH and an increased LH:FSH ratio in obese infertile patients. Further, the authors concluded that weight loss has been demonstrated to lead to a reduction in LH levels in patients with polycystic ovary syndrome (PCOS) but did not alter the pulsatility of LH secretion [17]. Also, Bohlke et al. assessed the relationship between BMI and basal LH and the LH: FSH ratio and found an inverse association between BMI and basal LH levels [18]. Sathya et al. did not find a relation between obesity and basal FSH [16]. Generally, LH:FSH ratio is still a very controversial criterion for identifying only a sub-group of infertile women with PCOS and abnormalities at the level of the HPO axis [19].Prolactin has been proposed to play an important role in the pathophysiology of obesity. Even mildly obese women have been found to display an enhanced prolactin secretion across the 24-h cycle as compared with normal-weight women. When examining the possible effect of obesity on elevated prolactin, which should be, spontaneous prolactin release has been shown to be significantly elevated in obese women in direct proportion to the size of the visceral fat mass. Because prolactin is inhibited by activation of the dopamine D2 receptor, increased prolactin secretion may occur due to reduced D2 receptor availability in the brain, which makes these individuals more likely to have elevated prolactin secretion [20]. In agreement, our results showed that subjects with morbid obesity had significantly higher levels of prolactin compared to other groups; average weight 14.81±6.68 ng/ml, overweight 15.47±7.92 ng/ml, obese 16.19±9.25 ng/ml and morbid obesity 19.12±8.73 ng/ml (p<0.05).Our study also showed that the lower the BMI the higher the U/S evidence of mature follicles; average weight 86.9% of follicles were mature, overweight 61.1%, obese 42.9% and morbid obesity 33.3% (p<0.05). There are also several studies that agree with our results and concluded that there is a definite relationship between anovulation and BMI [5, 21].Our results may be attributed to abnormal leptin levels in obese patients which is believed to play a role in ovarian folliculogenesis. Leptin participates in regulation of ovarian folliculogenesis indirectly via control of LH and FSH secretion. More recent evidence suggests that leptin also has direct regulatory actions on the developing follicle. The presence of leptin receptors on follicular cells, including oocytes, and early pre-implantation embryos suggests that leptin may play a direct physiologic role in follicular maturation, oocyte development. Because circulating leptin levels are directly related to body adiposity, elevated leptin concentrations associated with obesity may partly explain the negative impact of obesity on ovulation through its effect on FSH secretion and its direct effect on the follicles [4, 22].There are several biological mechanisms through which obesity may increase the risk for anovulation. These mechanisms center on the HPO axis, which regulates both the menstrual cycle and ovulatory function through a complex hormonal regulation system. The two main disturbances to the HPO axis occur through either hyperandrogenism or insulin resistance. Hyperandrogenism is a biological condition where there is an excess production or secretion of androgens, which include sex hormones [23]. Adipose tissue has been shown to have the potential to alter the secretion of sex hormones, given its essential role in both androgen production and in the conversion of androgens into other sex hormones [21]. In the same direction, insulin resistance and hyperinsulinemia can lead to disturbance in ovulatory function. The ovary is a target organ for insulin to stimulate the production of sex hormones. This increased insulin level will negatively affect follicular maturation through increasing free insulin-like growth factor 1 [9, 24].
5. Conclusions, Recommendations and Future Aspects of Research
- In conclusion, obesity is suggested to have a negative impact on the dynamics of ovulatory hormones, quality of oocyte and thickness of endometrium; and therefore may reduce fertility. So, nurses and nutritionist should be widely included in programs that could help the infertile women reduce their weight and consequently get a higher chance of ovulation. Further, extra research should focus on the role of weight reduction on ovulation and whether special diets are only enough to achieve the hormonal changes needed for ovulation or sport practice should be also taken into consideration. Health educational programs should be commenced to raise awareness about the negative impact of obesity on ovulation and fertility. Such programs should not only focus on infertile women but should include health care givers as well.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML