-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Nursing Science
2012; 2(3): 14-22
doi: 10.5923/j.nursing.20120203.01
Cognitive Protection by Angiotensin Converting Enzyme Inhibitors in Heart Failure
Ponrathi Athilingam 1, Cindy Munro 2, Rita D’aoust 3, Amy Karch 4, Leway Chen 5
1ACNP, FAANP, College of Nursing, University of South Florida, Tampa, 33612, Florida
2APRN, FAAN, College of Nursing, University of South Florida
3ANP-BC, CNE, FAANP, FNAP, College of Nursing, University of South Florida
4MS, RN, School of Nursing, University of Rochester, Rochester, 14642, NY
5MPH, FACC, Department of Cardiology, University of Rochester, Rochester, 14642, NY
Correspondence to: Ponrathi Athilingam , ACNP, FAANP, College of Nursing, University of South Florida, Tampa, 33612, Florida.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
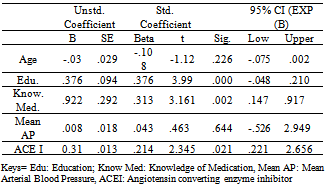
Cardio-selective medications such as angiotensin converting enzyme (ACE) inhibitor centrally active or non-centrally-active and angiotensin receptor blocker (ARB) have been researched in animals and humans for potential cognitive benefits. This secondary analysis presents results of a published cross sectional study of 90 community-dwelling adults with heart failure (HF) who were screened for cognitive impairment using the Montreal Cognitive Assessment (MoCA). The mean MoCA score was 24.73 (SD 2.76). T-test on the MoCA score and ACE inhibitor (78% of participants) and centrally-active ACE inhibitor (60%) were statistically significant (p= 0.01) with no association to ARBs. Bivariate analysis indicated higher dosages of ACE inhibitors were associated with better cognitive function (R= 0.211, p=0.046) with no association to ARBs. In the multiple regression model, covariates age, education, knowledge on HF medication, and mean arterial blood pressure accounted for 33.6% of variance and ACE inhibitor added 4.1 % of variance and remained statistically significant (p=0.021); age and mean arterial blood pressure were not significant in the final regression model. Findings from this study provided rationale to support the protective role of ACE inhibitors on cognitive decline among HF patients.
Keywords: Heart Failure, Angiotensin Converting Enzyme Inhibitors, Centrally Active ACE Inhibitor, Cognitive Impairment, Montreal Cognitive Assessment
Article Outline
1. Introduction
- Heart failure (HF) is a progressive disorder that occurs as a cumulative consequence of all affronts to the heart over one's life. HF is becoming increasingly common, with a high burden of morbidity and mortality; and has an enormous financial impact which is primarily associated with frequent readmissions[1].Approximately 50% of HF readmissions have been attributed to lack of knowledge and adherence to self-care recommendations[2]. HF self-care management requires that patients recognize a change (such as increasing edema), evaluate the change, decide to take action (e.g., take an extra diuretic dose) and implement a treatment strategies prescribed[3]. For many persons with HF, however, cognitive impairment may interfere with their ability to comply with HF therapy and perform adequate self-care, which contributes to increased incidence of morbidity, mortality, and higher readmission rates[4,5,6]. Research shows that cognitive impairment, particularly declines in executive function and memory, is a growing problem in HF management[7-10]. Its prevalence in persons with HF ranges from 25% to 75%[11,12] and have a 4-fold greater risk than the general population of having mild cognitive impairment[13]. In a 9-year follow-up of a community-based cohort of 205 HF patients, HF was associated with a multi-adjusted hazard ratio (HR) of 1.84 (95% confidence interval (CI) 1.35-2.51) for dementia and an HR of 1.80 (95% CI 1.25-2.61) for Alzheimer’s disease[14]. Given the serious impact of cognitive deficits on HF management, researchers have begun looking for therapies that may help protect cognition while improving HF outcomes.Hence, a secondary analysis was performed on a cross-sectional data set to examine benefits of cognitive protection using angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blocker (ARB) in HF.Current HF management includes drugs with various vascular effects that are identified as successful interventions in improving survival[15]. Cardio-selective medications such as ACE inhibitors, ARBs and beta blockers are considered life-sustaining drugs because they can prevent remodelling of the heart and thus reduce mortality and morbidity[17-19].Animal studies suggest cognitive protection of ACE inhibitors[18,20]. In humans, however, the effects of ACE inhibitors on cognition are controversial[21]. Zuccala and colleagues found that use of ACE inhibitors among patients with heart failure (N=1220) was associated with improving cognition (odds ratio=1.57; 95% CI 1.18-2.08)[22]. The probability of improving cognitive performance was higher for dosages above the median values, as compared with lower doses (odds ratios=1.90 and 1.42; p= .001), and increased with duration of the treatment (odds ratios for the lower, middle, and upper quartiles (1.25, 1.34, and 1.59; p= .007)[22]. This was supported in a study of 74 older adults that ACE inhibitors provided stability of cognitive function[23]. However, a recent animal study failed to demonstrate an effect on cognitive protection with the use of ACE inhibitor and ARBs[24].
2. Physiology of ACE Inhibitors, ARBs and Cognitive Protection
- A complex system of neuroendocrine interactions protects the heart, endothelium, brain, and kidneys from physiologic variation[16,17]. The Renin-Angiotensin System (RAS) regulates the vascular response to injury and inflammation[25.26]. Repeated response to injury and inflammation leads to endothelial dysfunction and microvascular damage[27]. In addition, the brain possesses an intrinsic RAS that is involved in memory and cognition[28,29].Although specific mechanisms are unclear, stimulation of the RAS has been linked with the activation of inflammatory cytokines that may play a role in degenerative dementias[30].The Health ABC study provided evidence that ACE inhibitors may have additional cognitive protection for executive function, but only in a select group of elderly Caucasians with angiotensinogen gene polymorphisms known to be associated with increased RAS system activity[31].Renin is responsible for the conversion of angiotensinogen to angiotensin I and provides additional substrate for ACE-mediated conversion to active peptide angiotensin II[32].This conversion takes place primarily on the vascular endothelial surface of the lungs with some local formation in the peripheral vasculature, and increases the availability of bradykinin[33,34]. ACE inhibitors block the conversion of angiotensin I to angiotensin II. Angiotensin II has been implicated in disruption of memory and recall, since it was attenuated by central infusion of captopril in rats[33].It has also been reported that angiotensin II injected into the dorsal neostriatum five minutes following passive avoidance conditioning interfered with the recall of the conditioned response 24 hours later in rat models[34] and mouse models[35,36]. In addition to their presence in peripheral circulation, both angiotensin II and ACE (in the form of ACE2) are present in the brain, with the highest densities in the striatum of the brains of rats[37] and mice[38,39,40]. The mechanism of ACE2 in the brain on cognitive protection has been primarily examined in animal models and warrants further exploration in HF. Figure 1 below illustrates the pathways of the RAS system.
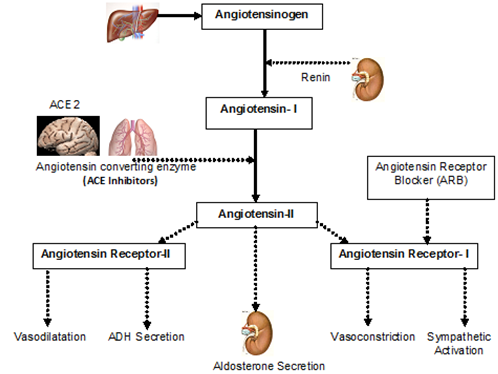 | Figure 1. Schematic illustration of the Renin-Angiotensin System (RAS) |
3. Methods
3.1. Design and Sample
- A secondary analysis was conducted on data from a published cross-sectional study of 90 community-dwelling adults aged 50 and above with a clinical diagnosis of HF as defined by the ICD-9-CM[47].Persons were included if they were in NYHA classification groups I-III and American Heart Association HF stage B,C, or D. Persons were excluded if they were: on continuous oxygen; listed for heart transplant with United Network for Organ Sharing status 1A or 1B; supported by a ventricular-assist device or home inotropic therapy; enrolled in a palliative or hospice care program; clinical diagnosis of dementia or Alzheimer’s Disease; and clinical history of stroke due to high-risk multi-infarct dementia. The primary study used the MoCA as a screening tool in patients with HF because it was found to be more sensitive than other tools[47].This study was approved by the human subject review board and participants were recruited from cardiology clinics affiliated with the University of Rochester by the PI. Eligible participants were consented and identified with a code number.
4. Measures
4.1. Cognitive Function
- Cognitive function (the primary outcome of interest) was measured using the MoCA[48]. The MoCA, a simple cognitive screening tool has eight cognitive domains that are most commonly affected in heart failure patients: 1) visuo-spatial/executive function, 2) naming animals, 3) memory, 4) attention, 5) language, 6) abstraction, 7) delayed recall, and 8) orientation. The MoCA has a total score range of 0-30 and a final total score of 26 and above is considered normal, a score of 22 to 25 is classified as mild cognitive impairment, 17 to 21 is moderate cognitive impairment, and score below 17 is considered dementia. The scores from memory domain (0-1) were not included in the total score but were used to measure short term memory (score of zero indicates that person was unable to remember the 5 items in two trials). The MoCA was developed to screen older people who present with mild cognitive impairment but who usually perform within the normal range on the MMSE[49]. It has been validated and approved as a screening tool for mild cognitive impairment by the Canadian Guidelines for the Diagnosis and Management of Dementia with a Cronbach’s alpha .83[49]. It has also been recently validated as a screening tool for cognitive function in patients with stroke[50] and cardiovascular disease[51]. A recently published study found that 70% of HF participants had mild cognitive impairment identified by the MoCA[52]. The MoCA incorporates the clock drawing test (which has been reported as being 50% more sensitive among HF participants)[53]. The MoCA involves a paper and pencil test in a question and answer format conducted by the interviewer. To minimize the ceiling effect of education, one point is added to the MoCA total score for participants with an education of high school or below[48].
4.2. Heart Failure Medications
- Data on HF medications including ACE inhibitors, ARBs, beta-blockers, aldosterone inhibitors, and HMG-CoA reductase inhibitors (statins) were obtained from patients and verified by chart review. ACE inhibitors were further classified as centrally-active or non-centrally-active ACE inhibitors and examined for dose association with cognition.
4.3. Demographic and Clinical Variables
- Data were obtained using a demographic and clinical questionnaire created for the study to describe the sample. Clinical variables, including ejection fraction, HF stage, HF medications, knowledge on HF medications, and etiology of HF were obtained from cardiologists’ records within the prior 6 months, and New York Heart Association (NYHA) functional classification was determined based on patients’ symptoms on the day of the study by a cardiology nurse practitioner. Depression which is highly associated with cognitive impairment was measured using GeriatricDepression Scale-15 (GDS-15),[54] comorbidity data by Modified Cumulative Illness Rating Scale,[55] and functional status was measured by Six-Minute Walk Test[56].
5. Data Collection and Analyses
- The demographic and clinical data questionnaire was administered first to develop rapport with patients, followed by other questionnaires and measures required for the primary study. Means and standard deviations were calculated for continuous variables. Frequencies were calculated for categorical variables. Bivariate analyses were performed to ascertain associations between variables of interest and cognitive function measured by the MoCA. T-test was used to compare differences on total MoCA score, and MoCA domain scores of those who were on ACE inhibitors and ARBs.
|
6. Results
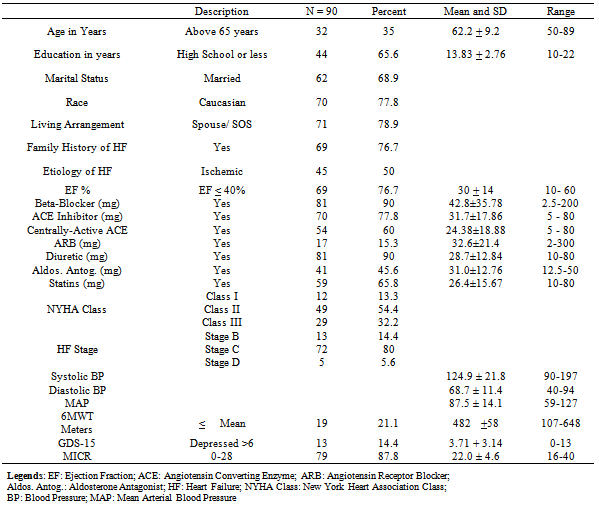
- The participants were mostly Caucasian (78%), mean age 62 years with SD+ 9 years (age ranged 50 to 89), male (66%), and married (69%). About 77% had low ejection fraction (≤40%). Ninety three percent of participants had prescriptions for either an ACE inhibitor or ARB; 77.8% (n = 70) were on ACE inhibitors, and 60% (n = 54) of those were on centrally-active ACE inhibitors. For consistency, ACE inhibitor and ARBs dosage was determined based on clinical equivalence dosing. See table 1 for details on demographic and clinical variables.
6.1. Cognitive Score Associated with ACE Inhibitors
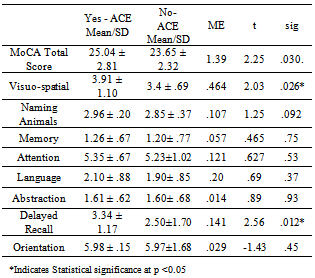
- We hypothesized that patients with HF who had a prescription for ACE inhibitors would have better cognitive function with higher MoCA total score and MoCA domain scores. This hypothesis was tested using an independent T-test to differentiate cognitive scores among the two groups. Levene’s test for equality of variance was assumed where indicated. As indicated in table 2, results were statistically significant for MoCA total score (t = 2.25, df 88; p=.03). Although, the mean scores on all MoCA domains were significantly different among groups, visuo-spatial domain (t =2.03, df 88; p= .026) and delayed recall (t = 2.56, df 88; p= .012) were statistically significant indicating that those who had a prescription for ACE inhibitor had scores that were significantly above the mean score on these domains.
|
|
|
6.2. Cognitive Scores and Centrally-Active ACE Inhibitors
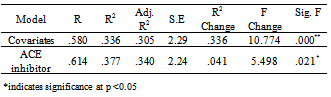
- ACE inhibitors were further classified as centrally-active or non-centrally active. Among this sample 60% were on centrally-active ACE inhibitors. T-test indicated significant association between total MoCA score and centrally-active ACE inhibitor (t =2.56; p <.013) with similar results on MoCA domains visuo-spatial and delayed recall (p <.05). However, regression model on centrally-active ACE inhibitor added 1% variance on the model which was not statistically significant (p .285).
6.3. Blood Pressure Associated with ACE Inhibitors
- Bivariate analysis indicated strong association between systolic blood pressure and ACE inhibitor (rS .272, p .001) and mean arterial blood pressure (rS .249, p .018) with significant association on dosage. A total of 59% of the participants had a history of hypertension. Having a history of hypertension and a prescription for an ACE inhibitor or ARB had no significant association with cognitive function measured by the MoCA in this sample of participants. In addition, when entered as a covariate, regression model did not support the association between mean arterial blood pressure and MoCA score (t = .463, p .644).
6.4. Cognitive Score and association with other Heart Failure Medications
- The MoCA total score was not associated with ARBs, aldosterone inhibition therapy, and statin therapy. However, the MoCA domain score on visuo-spatial (RS .23, p .032) and short term memory scores (RS .27, p .011) were strongly associated with aldosterone inhibition therapy.
7. Discussion
- Our data confirm previous findings that ACE inhibitors may have cognitive benefit among HF patients[22].Among study participants, cognitive function on total MoCA score was strongly associated with taking any ACE inhibitors and centrally-active ACE inhibitors with significant dose association, a finding supported by several researchers [22,23,44,45]. In addition, significant association was identified on visuo-spatial and delayed recall domains that have been reported as common domains of impairment in HF. It has been well documented that ACE inhibitor therapy leads to HF symptom improvement, reduced hospitalization and enhanced survival in patients with HF [15,17,21].Cognitive function may have direct impact on HF patients’ ability to perform self-care management at home [2-4,7]Recently, among 250 veterans, cognitive impairment was associated with poor adherence to medication regimen (p .007)[57]. Result from this study supports the hypothesis that ACE inhibitors may protect cognition in persons with HF and may thus improve adherence, a hypothesis that needs further exploration.On the contrary, no association was identified on ARBs alone among this sample of participants. This was contrary to the findings of Li and colleagues, who compared ACE inhibitor, ARB, and combination therapy among 819, 491 predominantly male (98%) US Veterans and reported a dose-response as well as additive effects on cognition from combination therapy with an ACE inhibitor and an ARB[58]. Li and colleagues also reported that combination therapy (ACE plus ARB) was associated with a reduced risk for dementia over 4 years (p= .016) compared with ACE inhibitors alone.58 Among participants with pre-existing Alzheimer’s disease, combination therapy (ACE plus ARB) showed a lower risk of admission to a nursing home (p .0001)[58].Our sample (N=90) had a total of 17 (15.3%) participants who were on ARB alone and 11 participants (9.9%) on both ACE inhibitor and ARB. This fairly small sample of participants may explain the non-association with cognitive function with combination therapy in our sample. Although there was no association between total MoCA score, ARBs and other cardiac medications, the MoCA domain visuo-spatial and short term memory scores were associated with aldosterone inhibition therapy. Particularly, draw-a-clock test, a single item that tests visuo-spatial function was statistically significant with prescription for ACE, and aldosterone inhibition therapy, a finding supported by Riegel and colleagues that draw-clock-test was 50% more sensitive in HF[53]. There is increasing evidence that hypertension may contribute to the development of cognitive impairment and that blood pressure lowering therapy might protect against cognitive deterioration and vascular dementia [15-17,59,60]. Although, bivariate analysis revealed association between MoCA score and systolic blood pressure, the findings was not supported by the regression model. In addition having a history of hypertension and a prescription for an ACE inhibitor or ARB had no significant association with cognitive function measured by the MoCA in this sample of participants, warranting further exploration. A total of 93% of participants in this study had a prescription for an ACE inhibitor and/or ARB, which is congruent with National data from the CMS quality index[61]. A major limitation of this study is the lack of a control or comparison group. The MoCA was used as a screening tool for mild cognitive impairment, and we did not obtain a gold standard neuropsychological test battery to more thoroughly evaluate cognitive function. HF diagnosis was ascertained using ICD-9 codes that may have introduced inaccuracies [62].
8. Summary and Implications for Nursing Practice
- ACE inhibitors are an important component of standard HF therapy and may confer an added benefit by improving cognitive function. It has been demonstrated that a complex chain of events leads to irreversible cardiac damage as shown in figure 1; leading eventually to overt HF. Angiotensin plays a prominent role in many of these events and thus the interruption of these progressive deleterious events is vital is protecting end organs[63]. There is conflicting evidence about the potential benefits of ACE inhibitors for the treatment of cognitive protection whether in HF, or other chronic health conditions such as diabetes, stroke, Parkinson’s disease and Alzheimer’s disease. The results from this study added evidence that ACE inhibition has emerged as a potential modality of cognitive protection possible by minimizing irreversible damage to heart and brain. Evidence is also emerging on the selective use of ACE inhibitors in individuals who carry specific genotypes as it may offer additional cognitive benefit beyond lowering vascular risk[64]. Future research on ACE genotypes may substantiate the use of ACE inhibition in clinical application for avoiding or minimizing cognitive impairment.
9. Conclusions
- The results suggest that ACE inhibition occupies a pivotal role in protecting end organs and are well-tolerated by most individuals. The prescription of ACE Inhibitors protects the vascular wall against various injuries and maintains blood flow to the brain and peripheral vasculature and thus may be a protective factor for cognitive deterioration in older adults with HF and other comorbid conditions such as hypertension and diabetic.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML