-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2024; 13(1): 10-15
doi:10.5923/j.nn.20241301.02
Received: Aug. 10, 2024; Accepted: Aug. 27, 2024; Published: Aug. 30, 2024

Antioxidant Properties of Biogenic Silver Nanoparticles Obtained from Discarded Shell of Velvet Tamarind (Dialium cochinchinense) Fruit
Bright Ankudze
Department of Chemistry Education, University of Education, Winneba, Ghana
Correspondence to: Bright Ankudze, Department of Chemistry Education, University of Education, Winneba, Ghana.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

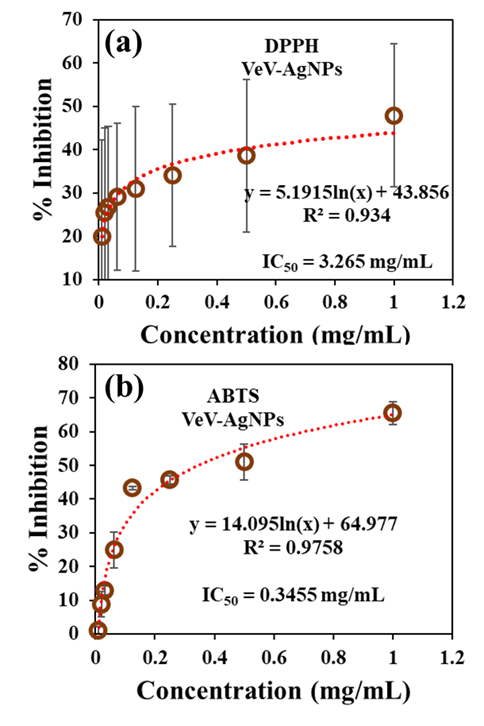
In this study, silver nanoparticles prepared from discarded shell of velvet tamarind fruit was investigated for its ability to scavenge for DPPH and ABTS free radicals. Silver nanoparticles prepared with such nanomaterial, which is considered a ‘waste’ is of great importance as it opens conversation on the exploration of new, cost effective, environmentally friendly bio-materials for antioxidant application. Extracts from the shells of velvet tamarind fruit upon interaction with silver ions led to the production of silver nanoparticles. The particles were characterized with Field emission scanning electron microscope, Particle X-ray diffractometer, UV-Vis spectrophotometer and Energy dispersive X-ray spectrometer to observe particle morphology, diffraction pattern, plasmonic absorbance and elemental composition, respectively. The antioxidant properties showed that the prepared silver nanoparticles (VeV-AgNPs) can actively scavenge free radicals with IC50 values of 0.3455 mg/mL and 3.265 mg/mL towards ABTS and DPPH free radicals, respectively.
Keywords: Silver nanoparticles, Velvet tamarind, Antioxidant, Green synthesis, ABTS, DPPH
Cite this paper: Bright Ankudze, Antioxidant Properties of Biogenic Silver Nanoparticles Obtained from Discarded Shell of Velvet Tamarind (Dialium cochinchinense) Fruit, Nanoscience and Nanotechnology, Vol. 13 No. 1, 2024, pp. 10-15. doi: 10.5923/j.nn.20241301.02.
Article Outline
1. Introduction
- The preparation of silver nanoparticles by green synthesis often involves the use of extracts from biological sources [1]. Plants extracts over the years are predominantly used for the preparation of silver nanoparticles due to their cost effectiveness and availability. Plant also possesses phytochemicals [2], which when used to prepare silver nanoparticles, are able to transfer some of the properties to the prepared particles which increases their scope of application [3]. As a result, biogenic nanoparticles prepared with plant extract are widely applied in nanomedicine as antimicrobials [4], antioxidants [5], drug delivery systems [6], diagnostic tool, medical imaging systems, and cancer treatments agents [7], etc. Biologically prepared silver nanoparticles over the years have exhibited great antioxidant potentials. Antioxidants play crucial role in reducing excessive free radical in a living system. Studies have shown that free radicals can be generated in a living system through metabolic activities, and serve as a defensive mechanism for the body [5]. It has also been reported that smoking cigarette [8], taking certain medication, external factors such as pollution [9] and exposure to radiation can lead to the accumulation of free radicals in the body. Free radicals in a living system (reactive nitrogen and oxygen species) can lead to oxidative stress when the antioxidant defence of a cell is much lower [10]. When exposed to living cell, free radicals are able to oxidize double bonds of certain fatty acids in lipids leading to the formation of aldehydes and peroxides [11]. This process alters the cell membrane structure and hence its permeability, thereby, affecting the transport of certain ions, causing protein inactivation and modifying enzymatic activities [11]. The alteration of cell structures as a result of oxidative stress can lead to diseases such as cancer, atherosclerosis, Alzheimer’s and cardiovascular diseases [12-14]. Over the years there have been intense search for non-toxic and effective antioxidants. Antioxidants come in natural forms such as ascorbic acid [15], beta-carotene [16], glutathione and alpha-tocopherol [17] or synthetic forms such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG) and tert-butyl hydroquinone (TBHQ) [18]. Most synthetic antioxidant, although active, are known to be less safe and can be toxic, as a result, studies have focused on the production of relatively cheap, effective and non-toxic antioxidants. Metal nanoparticles-based antioxidants over the years are being explored as potent antioxidant systems [19]. For metal nanoparticle-based antioxidant systems, biogenic silver nanoparticles are probably the most widely used compared to chemical or physically prepared particles due to their efficiency, eco-friendliness and non-toxicity. Biogenic silver nanoparticles are now prepared with leaf extract of plant such as Aesculus hippocastanum, Costus afer, Indigofera tinctoria, Clerodendrum inerme, Rhododendron dauricum, Pongamia pinnata, Tropaeolum majus L. Allium ampeloprasum L., Taraxacum officinale and Psidium guajava L. [5]. Vegetables such as cabbage and garlic, and fruit like Prosopis farcta when used to prepare silver nanoparticles have also shown potent antioxidant properties [5]. In the search for potent biogenic silver nanoparticles for antioxidant applications, extracts from edible, and photosynthetic parts of plant (leaves) have become the most common source of reductants. These plant parts play crucial roles, as they serve as food sources, [20] leaves on the other hand play critical role in the fight against global warming [21]. Therefore, studies have focused on the use of other ‘less important’ plant parts for the production of biogenic silver nanoparticles for antioxidant applications. Silver nanoparticles produced with plant parts such as peels of Ipomoea batatas have exhibited effective antioxidant properties [22]. In the present study, we explore silver nanoparticles prepared with extract from discarded shell of velvet tamarind as effective antioxidant. Velvet tamarind is a tropical fruit known for treating ulcers and heart related diseases [23-25]. The fruit is encased in a shell which is often discarded. In our previous study, extract from the shells of velvet tamarind was used to prepare silver nanoparticles in a cost effective, simple and efficient manner. The obtained silver nanoparticles were studied for their antimicrobial synergistic potentials and the surface-enhance Raman scattering properties [26]. In the present study, the antioxidant properties of the prepared particles are reported. The ability of the Velvet tamarind stabilised silver nanoparticles to scavenge ABTS and DPPH radicals were studied. The results reveal that silver nanoparticles obtained from the shells of velvet tamarind can serve as a potential nanomaterial for antioxidant applications.
2. Experimental
2.1. Chemical
- The silver precursor used for the synthesis of velvet tamarind extract stabilized silver nanoparticles was silver nitrate (AgNO3, Merck, ≥ 99%). The Velvet tamarind fruit from whose shell the extract used as a reducing agent was prepared was obtained from the local market in the central region of Ghana. DPPH (1,1-diphenyl-2-2-picrylhydrazyl), potassium persulphate and ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) were used in the study of the antioxidant properties of the prepared silver nanoparticles.
2.2. Synthesis of Silver Nanoparticles of Silver Nanoparticles Prepared from Discarded Shell of Velvet Tamarind
- In the synthesis of silver nanoparticles, the extract solution was first prepared. About 2.5g of shells were first washed with hypochlorite solution. Deionized water (40 mL) was added and left overnight. A resulting pale-yellow solution was obtained, the shells were separated, the solution filtered and stored as the reductant for silver nanoparticles preparation. About 2 mL of AgNO3 was added to 40 mL of the extract solution. The mixture was gently stirred and exposed to sunlight. The reduction of the silver ion leading to the formation of silver nanoparticles was observed as a colour change from pale-yellow to dark red.
2.3. Characterization
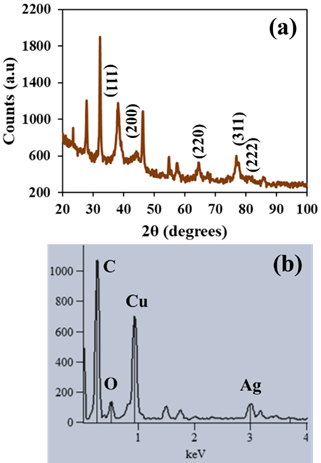
- Morphological study of the VeV-AgNPs was done with Hitachi S-4800 FE-SEM (field emission scanning electron microscope). The diffraction pattern of the prepared silver nanoparticles was acquired with PANalytical Empyrean X-Ray Diffractometer. Energy dispersive X-ray spectrometer (EDS) operating on a Noran system six (NSS) software was used for elemental analysis of the VeV-AgNPs, whereas Shimadzu UV - 1800 UV-VIS Spectrophotometer was used for the measurement of the plasmonic absorbance of the prepared silver nanoparticles. Drawell DNM-9602 microplate reader was used to read absorbances during the antioxidant studies.
2.4. ABTS (2,2'-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) Radical Scavenging Activity
- To investigate the ABTS scavenging activity of the VeV-AgNPs, free radicals were first created. About 10 mL of 2.4 mM potassium persulfate and another 10 mL of ABTS (7 mM, 10 mL) solution were prepared in distilled water. A mixture of these two solutions served as the stock solution and was incubated at room temperature in the dark for 12 hours before use. A working solution was prepared by diluting 1 mL of ABTS stock in 9 mL of distilled water. The VeV-AgNPs was prepared into different concentration through serial dilution into 1.0, 0.5, 0.25, 0.125, 0.625, 0.313 mg/mL etc. About 40 µL of the different VeV-AgNPs concentration was pipetted into microplates containing 160 µL of ABTS working solution. The plates were incubated for 30 minutes in the dark, followed by the measurement of absorbance at 734 nm. The same was done using ascorbic acid as the test sample. The ABTS scavenging activity was determined from the following relation:
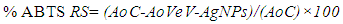 | (1) |
2.5. DPPH (1,1-Diphenyl-2-2-Picrylhydrazyl) Radical Scavenging Activity Assay
- In the DPPH assay, a 0.1 nM working solution was prepared by adding 1 mL stock solution to 9 mL of methanol. An amount of 160 µL of DPPH working solution was pipetted into a 96 well microplate. The VeV-AgNPs was prepared into different concentration of 1.0, 0.5, 0.25, 0.125, 0.625, 0.313 mg/mL etc. through serial dilution. About 40 µL of the different VeV-AgNPs concentration was pipetted into microplates containing 160 µL of DPPH working solution. The plates were incubated in the dark for 30 minutes. Afterwards, the absorbance was measured at 517 nm using a microplate reader. The DPPH scavenging activity was determined from the following relation:
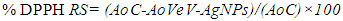 | (2) |
3. Results and Discussion
3.1. Morphological Study of Silver Nanoparticles (VeV-AgNPs)
- The average diameter of the VeV-AgNPs observed under the FE-SEM microscope was 30.0 ± 8.5 nm as presented in Figure 1(a). Particles were mostly quasi-spherical; however, rod-like nanoparticles were occasionally observed. These rod-like particles have aspect ratio of about 33:21 nm. Silver nanoparticles have plasmonic properties due to their ability to generate surface plasmon resonance (SPR). SPRs are produced due to the oscillation of electrons in the conduction band of silver nanoparticles. The generation and observance of SPRs may indicate the production manometer scale silver particles [27]. The plasmonic properties of the prepared VeV-AgNPs was ascertained by measuring their absorbance using UV-Vis spectrophotometer. As shown in Figure 1(b), the plasmonic absorbance of the VeV-AgNPs were observed around 455 nm in the visible region of the electromagnetic spectrum.
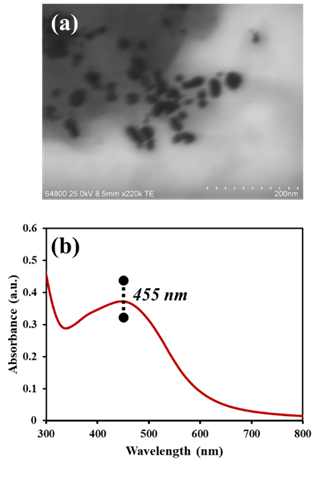 | Figure 1. (a) STEM image of VeV-AgNPs showing a quasi-spherical and rod-like particle morphologies, (b) UV-Vis spectrum of the VeV-AgNPs showing a plasmonic absorption maximum around 455 nm |
3.2. Antioxidant Properties of VeV-AgNPs
- The antioxidant properties of the synthesized VeV-AgNPs were studied using two different assays, namely DPPH and ABTS assay. These are spectrophotometric methods and are based on the interaction between coloured radical and antioxidant sample. In the first assay, the working DPPH radical solution was prepared in methanol. Figure 3 shows the structure of a typical DPPH free radical. The molecule is stabilized due to the spare electron which is delocalised over the entire molecule [5]. The delocalization of the electron enables the DPPH radical to resist dimerization, hence its stability. It is also responsible for the observed dark purple colour of the molecule, as well as, the UV-Vis absorption maximum around 517 nm in the visible region of the electromagnetic spectrum. To study the ability of the VeV-AgNPs to scavenge DPPH free radical, the sample was added to the radical molecule following by the measurement of absorbance [5].
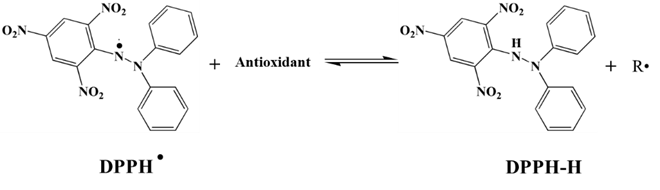 | Figure 3. DPPH free radical generation and its reaction with antioxidant |
 | Figure 4. Scavenging activity of VeV-AgNPs against (a) DPPH free radicals (b) ABTS free radicals |
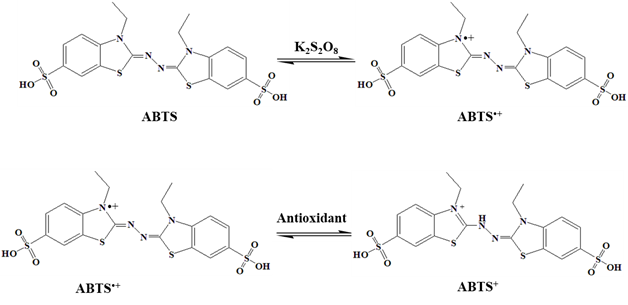 | Figure 5. ABTS radical generation and its reaction with antioxidant |
4. Conclusions
- This study focused on the antioxidant properties of silver nanoparticles obtained from the shells of velvet tamarind fruit (VeV-AgNPs). A simple, cost effective and sustainable method was employed to obtain the silver nanoparticles. Two different assays, namely, DPPH and ABTS scavenging assay were used to study the antioxidant properties of the obtained silver nanoparticles. The results from the scavenging assay reveal that the prepared VeV-AgNPs possess antioxidant properties. The scavenging activity of the VeV-AgNPs towards DPPH and ABTS free radicals revealed an IC50 values of 3.265mg/mL and 0.3455 mg/mL, respectively.
ACKNOWLEDGEMENTS
- Christopher Dawari of University of Eastern Finland is acknowledged for the assistance he rendered in the measurement of STEM images.
Conflict of Interest
- The author declares no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML