-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2023; 12(1): 1-6
doi:10.5923/j.nn.20231201.01
Received: Oct. 26, 2023; Accepted: Nov. 22, 2023; Published: Nov. 29, 2023

Surface-Enhanced Raman Scattering (SERS) Properties of Velvet Tamarind (Dialium cochinchinense) Stabilized Silver Nanoparticles
Bright Ankudze1, Francis Nsiah2
1Department of Chemistry Education, University of Education, Winneba, Winneba, Ghana
2Department of Chemistry, School of Physical Sciences, University of Cape Coast, Cape Coast, Ghana
Correspondence to: Bright Ankudze, Department of Chemistry Education, University of Education, Winneba, Winneba, Ghana.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Surface-Enhanced Raman Spectroscopy (SERS) since the inception of its application has proved useful in revealing surface chemistries of various fabricated materials. Considerable effort is being made in the fabrication SERS-active platform based on green synthesized silver nanoparticles. Such approach is aimed at reducing toxicity associated with conventional substrate preparation using synthetic chemicals. Nonetheless, most of the bio-resources used in preparing SERS substrates by green synthesis are edible and photosynthetic plant parts, which in over-dependence, can be detrimental to the eco-system. In our previous study, a simple green synthesis approach which involves the use of discarded shells of velvet tamarind to prepare quasi spherical silver nanoparticles (VeV-AgNPs). The silver nanoparticles were studied by acquiring plasmonic absorption, X-ray diffraction pattern and scanning electron microscopy image with UV-Vis spectrophotometer, X-ray diffractometer and scanning electron microscope, respectively. In the present study, the SERS properties of the VeV-AgNPs was studied using 4-Aminothiophenol as a probe molecule. It was observed that the VeV-AgNPs on glass slide were able to detect the 4-Aminothiophenol molecule at a low concentration of 1×10-7 M with analytical enhancement factor of 1×104. The results from this study shows that silver nanoparticles prepared from the discarded shells of velvet tamarind can serve as a simple, cost-effective and eco-friendly way of preparing nanoparticles for SERS-based molecular sensing.
Keywords: Silver nanoparticles, Velvet tamarind, SERS, Green synthesis
Cite this paper: Bright Ankudze, Francis Nsiah, Surface-Enhanced Raman Scattering (SERS) Properties of Velvet Tamarind (Dialium cochinchinense) Stabilized Silver Nanoparticles, Nanoscience and Nanotechnology, Vol. 12 No. 1, 2023, pp. 1-6. doi: 10.5923/j.nn.20231201.01.
Article Outline
1. Introduction
- Raman spectroscopy is a non-invasive, non-destructive and highly specific spectroscopic technique that utilizes Raman scattered light for chemical fingerprinting [1]. When light incidents on a molecule, majority is scatter elastically, while tiny amount interact inelastically due to energy exchange between the photons and the molecule. Since inelastically scattered light emanates from the interaction of light with a molecule, it helps in material identification [2]. However, less than 1% of photons are scattered inelastically, thus making the Raman spectroscopic technique very weak and less sensitive. Over the years, there have been efforts to enhance the Raman scattered light emitted by a molecule through techniques such as Fourier Transform (FT) Raman [3], Resonance Raman [4], Surface-enhance Raman spectroscopy/scattering (SERS) [5] etc. Surface-enhanced Raman scattering (SERS) is by far one of the widely used technique to acquire the Raman spectrum of a molecule as it significantly enhances the Raman effect [6]. The SERS effect is observed when analyte molecule is adsorbed on the surface of noble nanometal, and it is attributed to two mechanisms, namely, electromagnetic enhancement mechanism and chemical enhancement mechanism [5]. The electromagnetic enhancement mechanism originates from the excitation of localized surface plasmon resonance. When light incidents on a noble metal nanoparticle, the electrons in the conduction band oscillates, when the frequency the electron oscillations matches that of the excitation light, surface plasmons resonance is observed [7]. Surface plasmon is associated with strong electromagnetic field which is able to enhance the Raman effect of analyte molecule [8]. The chemical enhancement effect, on the other hand, originates from charge transfer. As charges move from the highest occupied molecular orbital (HOMO) to lowest unoccupied molecular orbital (LUMO) between the noble metal and analyte molecule, the Raman effect is enhanced [5]. Among the noble metals, silver nanoparticles are widely used as SERS platforms primarily due to their wide absorption of light in the visible region of the electromagnetic spectrum, biocompatibility, and low background signal [9]. Silver nanoparticles for SERS based application can be synthesized through a top-down or bottom-up approaches [10]. In a top-down approaches, bulk silver metal is broken down into sizes in the nanometer scale with techniques such as colloidal milling and electrical dispersion [11]. The bottom-up approach, on the other hand, involves the bulking up of silver particles into nanometer scale sizes through approaches such as laser ablation [12], sonication and wet chemical synthesis [13]. Silver nanoparticles for SERS application are mostly prepared through wet synthetic method, an approach which requires a reducing and stabilization agents [5]. These reducing and stabilizing agents could be synthetic chemicals [14], phytochemicals or chemical obtained from animals [15]. Over the years, the synthesis of silver nanoparticles for SERS-based applications using phytochemicals or chemicals derived from animals, through an approach known as green synthesis, is on the rise. The drive towards green synthesis arose because most of the conventional synthetic chemicals used in preparing silver nanoparticles for SERS-based applications are toxic, resulting in nanoparticles which are harmful to the environment. Normally, SERS substrates are discarded after single use, thus, the use of toxic nanoparticles for such platforms can be detrimental to the environment. As a results, studies have focused on the use of plants such as Psidium guajava, Illicium verum, Moringa oleifera, Persia americana, Vitis califonica etc. [16–20], have been explored to produce environmentally benign nanoparticles for SERS-based applications. Conventionally, plant parts such as fruit and leaves are mostly used to prepare these particles. The heavy dependence on such plant parts and product, as the demand for biogenic silver nanoparticles rise, can raise food security and climatic concerns. Therefore, studies are focused on other bio-resources which are non-consumable and “less important” to the environment. Plant parts such as palm shells [21], citrus peels [22], etc., have proven effective as bioresource for the production of active SERS platforms.In our previous study, the shells of velvet tamarind, a bioresource often discarded was studied as potent bio-source for green synthesis of silver nanoparticles [23]. Velvet tamarind, tropical plant with enormous health benefits [24-26], which are consumed to treat ulcer and heart related illnesses. In the present study, the SERS properties of the velvet tamarind stabilized silver nanoparticles is investigated. The results from the study reveals that silver nanoparticles prepared from the shells of velvet tamarind fruit can serve as active and sensitive platform for SERS-based detection of molecules.
2. Experimental
2.1. Chemicals
- Silver nitrate (AgNO3, Merck ≥ 99%) was used as precursor in the preparation of silver nanoparticles. Velvet tamarind was purchased from the local market (Central Region, Ghana). The shell was removed and washed severally with hypochlorite solution. A 4-Aminothiophenol (Sigma Aldrich, ≥ 97%) was used as a probe molecule to study the SERS properties of the prepared silver nanoparticles.
2.2. Preparation of Reducing Agent from Shell Extract of Velvet Tamarind
- In the preparation of reductant solution, 2.5 g of velvet tamarind shells obtained from fruit of the plant was weighed, washed with hypochlorite solution and soaked in 40 mL deionized water, and left overnight. The liquid (pale yellow) is finally filtered out and kept as the reductant solution for the preparation of silver nanoparticles.
2.3. Preparation of Silver Nanoparticles from Shell Extract of Velvet Tamarind
- Silver nanoparticles was prepared according to the protocol reported by Ankudze et. al. (2023) [23]. Briefly, to 40 mL of the velvet tamarind shell extract solution, 2 mL of AgNO3 (0.01 M) was added. The resulting mixture was stirred and kept outdoor under sunlight. The exposure to sunlight led to color change from pale yellow to dark red, indicating the formation of silver nanoparticles. The nanoparticle was purified by centrifuging the solution and stored for subsequent use.
2.4. Preparation of Surface-enhanced Raman Scattering (SERS) Substrate
- The prepared silver nanoparticle (VeV-AgNPs) was centrifuged to concentrate the particles. About 0.5 µL were dropped on a glass slide and allowed to dry under ambient condition. Afterwards, 5 µl of 4-Aminothiophenol probe molecule of different concentration were pipetted and dropped on the nanoparticle spot on the glass slide, followed by SERS measurements (Figure 1).
 | Figure 1. Preparation of VeV-AgNPs SERS substrate |
2.5. Estimation of Analytical Enhancement Factor (AEF)
- Equation (1) was used in the estimation of the analytical enhancement factor (AEF) [27]
 | (1) |
2.6. Characterization
- The morphological study of the silver nanoparticles was done with Hitachi S-4800 FE-SEM (Field Emission Scanning Electron Microscope). Samples were dropped on a carbon tape prior to the electron image acquisition. A Shimadzu UV - 1800 UV-VIS Spectrophotometer was used for recording the plasmonic absorption. Aqueous sample of the prepared silver nanoparticles were poured into a covet and subjected to UV-Vis measurement using water as a control. PANalytical Empyrean X-Ray Diffractometer was used for recording the PXRD patterns of the prepared silver nanoparticles. Elemental analysis was done with Energy dispersive X-ray spectrometer operating on Noran system six software. A Renishaw® inVia Raman microscope operating on wireTM 3.4 software was used for SERS studies. An excitation wavelength of 785 nm, a power of about 3 mW, an integrating time of 10 s was used in the SERS measurement.
3. Results and Discussion
3.1. Morphological Properties of Surface-enhanced Raman Scattering-active VeV-AgNPs
- Figure 2(a) shows the STEM image of silver nanoparticles prepared from shell extract of velvet tamarind fruit (VeV-AgNPs). The nanoparticles obtained from the synthesis comprised particles with spherical morphology with average diameter of 30.0 ± 8.5 nm. In addition, particles with rodlike morphology with aspect ratio of 33: 21 nm were also observed occasionally. The plasmonic absorption of the prepared silver nanoparticles (Figure 2(b)) was observed around 455 nm in the visible region of the electromagnetic spectrum.
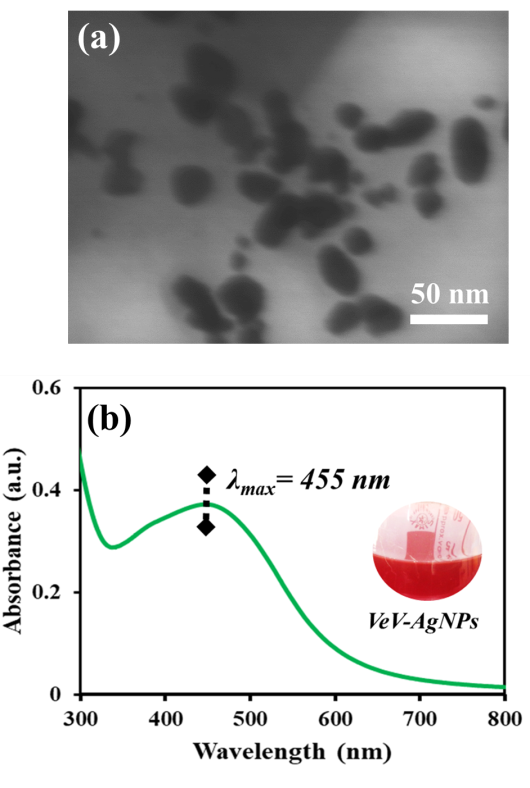 | Figure 2. (a) STEM image of VeV-AgNPs, (b), Uv-Vis spectrum of VeV-AgNPs |
3.2. Surface-enhanced Raman Scattering Properties of the VeV-AgNPs
- Figure 4 shows the SERS spectra of different concentrations of 4-Aminothiophenol acquired on the surface of VeV-AgNPs. As indicated in Figure 4(a), no characteristic peak of 4-Aminothiophenol was observed when the VeV-AgNPs were exposed to laser light without the 4-Aminothiophenol probe molecule. It is also noteworthy, that all the different concentration of 4-Aminothiophenol which were studied produced no characteristic peak of the molecule without the presence of the VeV-AgNPs, an indication that the concentration is very low and cannot be studied with normal Raman measurement. As presented in Figure 4(d), even at a low concentration of 1×10-7 M, the characteristic band of 4-Aminothiophenol could still be observed. The 4-Aminothiophenol molecule binds to the surface of silver nanoparticles via the sulphur atom. In most cases the C‒S and C‒C stretching bands are the widely reported bands which are characteristic of 4-Aminothiophenol [32]. When 4-Aminothiophenol was adsorbed on the surface of the VeV-AgNPs, these bands were observed around 1080 and 1592 cm-1 within the scan range of 1000 – 2000 cm-1.
4. Conclusions
- In this study, silver nanoparticles (VeV-AgNPs) prepared using extract from the shells of velvet tamarind as reducing agent was studied for their ability to serve as active surface-enhanced Raman scattering substrate. The VeV-AgNPs were deposited on a glass slide to dry, 4-Aminothiophenol was dropped on the nanoparticles and subjected to SERS measurement. Analytical enhancement factor to the order of 104 towards the 4-Aminothiophenol probe was estimated for the VeV-AgNPs substrate. The detection limit, as low as 1×10-7 M was determined for the VeV-AgNPs, an indication that it can serve as active platform for low concentration detection of molecules.
ACKNOWLEDGEMENTS
- The authors acknowledge the assistance of Christopher Dawari and University of Eastern Finland for measuring the SEM images of the silver nanoparticles.
Conflict of Interest
- The authors declare no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
