-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2022; 11(1): 1-6
doi:10.5923/j.nn.20221101.01
Received: Mar. 10, 2022; Accepted: Mar. 22, 2022; Published: Apr. 15, 2022

Ultrasound as a Good Technique for Obtaining Graphite and Graphene Nanosheets and the Influence of the Sonication Time Interval on the Dimensions of Graphite Nanosheets
Luiz Gustavo Barbosa1, Glaucia Karina Martofel1, Creusa Iara Ferreira2, Raquel Santos Mauler2
1Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Sul (IFRS), Campus Erechim, Erechim, RS, Brazil
2Instituto de Química, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
Correspondence to: Luiz Gustavo Barbosa, Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Sul (IFRS), Campus Erechim, Erechim, RS, Brazil.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article presents a brief review of some methods of obtaining graphite and graphene nanosheets, the latter a material that has attracted much attention from the scientific community in recent years, due to its different properties and possible applications. Next, the work addresses the subject of graphite exfoliation by the sonication method, which is simple and versatile, and by which it is possible to obtain graphite sheets with thicknesses of units of nanometers. Furthermore, the work presents experimental data that show the influence of the sonication time interval on the dimensions of graphite nanosheets. The results indicate that longer the sonication time interval, smaller the dimensions of the graphite sheets.
Keywords: Graphite Nanosheets, Graphene, Sonication
Cite this paper: Luiz Gustavo Barbosa, Glaucia Karina Martofel, Creusa Iara Ferreira, Raquel Santos Mauler, Ultrasound as a Good Technique for Obtaining Graphite and Graphene Nanosheets and the Influence of the Sonication Time Interval on the Dimensions of Graphite Nanosheets, Nanoscience and Nanotechnology, Vol. 11 No. 1, 2022, pp. 1-6. doi: 10.5923/j.nn.20221101.01.
Article Outline
1. Introduction
- The research area of materials science and engineering is constantly evolving, and among the most researched materials are those based on carbon, which in their different allotropic forms – graphite, diamond, fullerenes, nanotubes and graphene – can be manipulated. with the intention of improving their properties. Nevertheless, carbon-based materials - specifically graphene - when at the nano scale present differentiated and very attractive properties for the areas of engineering, electronics, energy, among others [1] due to their excellent thermal conduction (≈ 5000 W/ mK), optical transmittance greater than 98% [2], mechanical strength of approximately 130 GPa [3], mechanical stiffness of 1060 GPa [4] and modulus of elasticity of 1000 GPa [3]. Such properties could bring disruptive innovations when combined. For example, combining flexibility and transparency with its electronic property (high electrical conductivity) is possible to create flexible electronics. In other words, there would be a disruption, which could lead to a new industrial revolution [5]. Thus, the great potential of applications is evidenced, whether in improvements of existing technologies such as spintronics, as well in technologies that are under development, such as flexible electronics. But for this nanomaterial to be obtained and manipulated, it is necessary to employ specific techniques.The most used methods for obtaining carbon-based materials at the nanometer scale are: mechanical exfoliation of graphite, ultrasound, electrochemical exfoliation, chemical vapor deposition (CVD), physical vapor deposition (PVD), dissolution of graphite with superacid, alkylation of graphene derivatives, chemical reduction of organically treated graphene oxide, thermal exfoliation and chemical reduction of graphite oxide [6]. However, most of these methods are of high cost and/or high complexity, which makes it impossible to obtain them in some academic laboratories. However, techniques such as mechanical exfoliation, graphite arc discharge, thermal melting, phase exfoliation [7] and sonication are simpler methods capable of providing nanomaterials such as graphene [8], presenting the low cost as an advantage over the others techniques. In the last one – sonication – the equipment can be easily found in laboratories and is characterized by being a low-cost method, offering ease, versatility and speed, which justifies its study. Furthermore, ultrasound has been extensively used for the synthesis of materials over the years, and is now positioned as one of the most powerful tools for obtaining nanostructured materials [9,10]. The exploration of techniques using this device has been increasing, because in recent years the use of ultrasound in research related to graphene synthesis has become routine. In addition, the use of sonication solved problems related to the reaggregation of nanosheets and considerably reduced the synthesis time of this nanomaterial [11]. One study used polar aprotic solvents added to bath sonication, which favored a complete exfoliation, that is, obtaining one nano-thick graphite sheets [12], other studies also used sonication combined with aromatic solvents – hexafluoro benzene, octafluorotoluene, pentafluorobenzonitrile, pentafluoronitrobenzene, pentafluoropyridine and pyridine - which, in addition to facilitating the exfoliation of the graphite layers, facilitated the microscopic study by preventing the reaggregation of the nanosheets [13]. Another study used orthodichlorobenzene solvent (ODCB), evidencing the ability of sonication to guarantee solution stability [14].In a study with graphene-based composite materials and a sonochemical approach, a reactive solvent – styrene – was used for graphite exfoliation and as a monomer for the formation of reactive polymeric radicals. In this one, the importance of applying the ideal sonication time was observed, which provides a good exfoliation between the graphite layers [15].Other means of obtaining graphene through sonication are also in evidence. The single-step method, involving biphasic emulsion of double-distilled water (DDW) and dodecane, demonstrates the importance of sonication time in obtaining nanomaterials, as well in sample stability [16]. In this same line of research, there is a study with the application of sonication in emulsion stabilized by ionic liquids (ILs) - 1,3-dibenzyl imidazolium benzoate and 1-naphthalate - with different applied potencies, demonstrating the importance of sonication time versus power applied in obtaining few layers of graphene [17].Sonication applied to nanomaterials was also used to obtain nanosheets of molybdenum disulfide, similar to graphene, with the preparation of a solution of 1-dodecanethiol and chloroform, applied at different intervals of synthesis time, corroborating the other studies that report on the importance of the relationship between potency, time interval and exfoliation of a nanomaterial [18]. Other studies using sonication are the ones that applied this equipment as an adjunct, for example, combined with the Hummers method [19], in which it was also applied with dimethylformamide (DMF) to obtain graphene sheets on a sufficient scale to incorporate them homogeneously in the preparation of matrices [4]. The combination of sonification with other equipment can be also verified in works that apply the milling-ultrasonication method to prepare graphene nanosheets starting from the graphite flake. Sonication was used as one of the synthesis methods to help increase the exfoliation rate [20]. Also, in this line of equipment combination, aiming to increase the exfoliation rate, there is the combination of ultrasound in a high pressure atmosphere plus supercritical CO2 to exfoliate the graphite [21]. In this graphite was synthesized for the production of exfoliated films of graphene with pure polymer. Through this it can be affirmed that the greater the power, the greater the energy generated in the sonication and, therefore, the CO2 mass transfer is intensified between the graphite layers, facilitating its expansion and consequently the exfoliation [22].In the synthesis route aiming specific application, there is the use of sonication combined with heat treatment for graphene oxide / chitosan nanocomposite films. The films are intended for use in petrochemical-based food packaging, and with which there is yet another demonstration of the importance of controlling the sonication time in obtaining the sizes of nanosheets [23]. Next, there is a study on the preparation of films composed of polyvinyl alcohol (PVA) based on graphene, which requires uniform dispersion of graphene in the PVA added to an alignment of its structure. This also demonstrates one more benefit of the application of sonication [24].Based on the literature presented, it appears that there is an increase in the concentration of graphene as the sonication time increases [18]. In a study in which high-power ultrasonication was used in a dimethylformamide (DMF) solution, a high concentration of graphene nanosheets was obtained, with a yield ten times greater than if low-power sonication was applied [25]. Furthermore, if the graphite is prepared – expanded – and synthesized by means of sonication in dimethylformamide (DMF), it is easier to obtain nanolayers in relatively short time intervals [26].Thus, it can be seen from the studies presented that the ideal sonication points differ according to the fluid used and the type of sonicator equipment. Added to this are the facts that the evolution of ultrasonic fragmentation in 2D materials and the relevance of defining the time interval are essential to ensure good exfoliation, with quality of crystalline structures at the nanometer scale. In this context, the present work demonstrates the results obtained from the application of low power sonication, with ultrasonic bath equipment, in the exfoliation of graphite at different time intervals.
2. Materials and Methods
- Expanded graphite (GE) Micrograf HC-11 from Nacional do Grafite with an average particle size of 10 µm was used. The solvent applied was dimethylformamide (DMF) P.A. commercialized by Vetec Química Fina. Furthermore, 10 cm diameter polycrystalline silicon wafers and mica sheets were used.The granulometry of the graphite particles was obtained in a Cilas 1180 laser particle analyzer from the Laboratory of the Nucleus of Studies of Density Currents (NECOD) of the Federal University of Rio Grande do Sul (UFRGS). The equipment allows differentiating particles between 0.004 – 2500 µm.For application of the sonication method, three dispersions containing 1 ml of DMF with 1 mg of graphite were prepared and sonicated for periods of 1, 3 and 7 hours. The equipment used was a Unique USC 165 with a frequency of 25 kHz and a power of 75 watts.From the DMF/graphite dispersions, stirred in ultrasound, samples of 20 µL were collected and deposited on silicon dioxide substrates with an area of 1 cm2. For total solvent evaporation, the samples were submitted to the illumination of a 50-watt halogen dichroic lamp for 30 minutes. The spin coating method was also used to verify the size of the graphite particles after this process.Atomic force microscopy (AFM) were performed in a scanning probe microscope (SPM) of the Laboratory of Advanced Polymers and Nanocomposites of the Institute of Chemistry of UFRGS. The model 5500 AFM equipment, manufactured by Agilent Technologies, is equipped with a Pico Plus controller interface and operated using the Pico View software version 1.6.8. Image acquisition in intermittent contact mode was performed with conventional silicon tips with resonant frequencies of approximately 190 kHz, rod spring constant of 48 N/m and apex radius of less than 10 nm, manufactured by Nanosensors. The images obtained were analyzed using the WSXM program, version 5.0 developed by Nanotec. The length, width and height of the graphite particles dispersed on silicon dioxide and mica surfaces were measured.
3. Results and Discussions
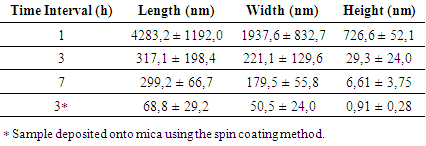
- The sonication time interval is the period for which a sample is subjected to ultrasound agitation. To study the influence of this parameter on the size of graphite particles, three solutions containing 1 ml of DMF with 1 mg of graphite were used. These solutions were subjected to sonication for periods of 1, 3 and 7 hours and, after that, samples of 20 µL of each solution were collected and deposited on silicon dioxide substrates for microscopy. Figure 1(a) shows an AFM topographic image, in intermittent contact mode, of a sample collected from the sonicated solution for 1 hour. In this image is possible to observe a large graphite particle whose topographic profile is shown in Figure 1(b). In this profile is possible to observe that the graphite particle has a length of approximately 5.5 µm and a height of almost 600 nm.
 | Figure 1. In (a) an AFM topographic image of a sample collected from the solution of DMF and graphite sonicated for 1 hour. In (b) the topographic profile of the particle shown in (a) |
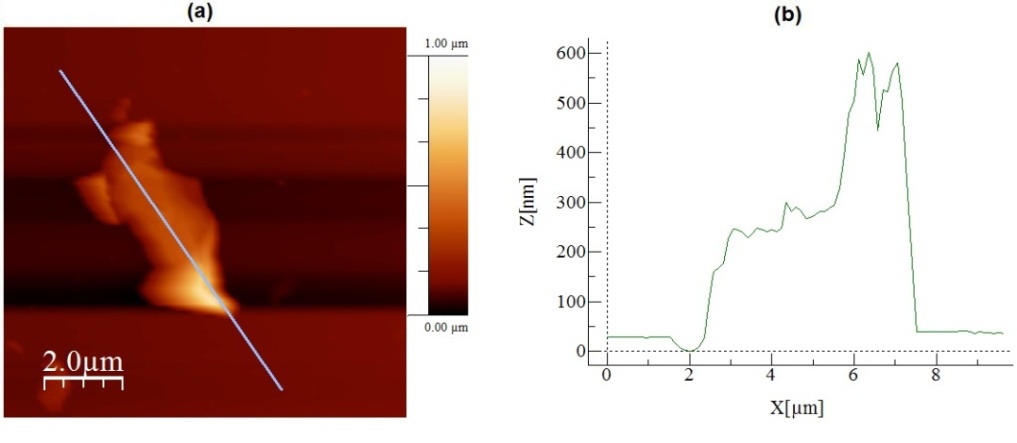
 | Figure 2. In (a) an AFM topographic image of a sample collected from the sonicated graphite and DMF solution for 3 hours. In (b) a topographic profile traced over the image presented in (a) |
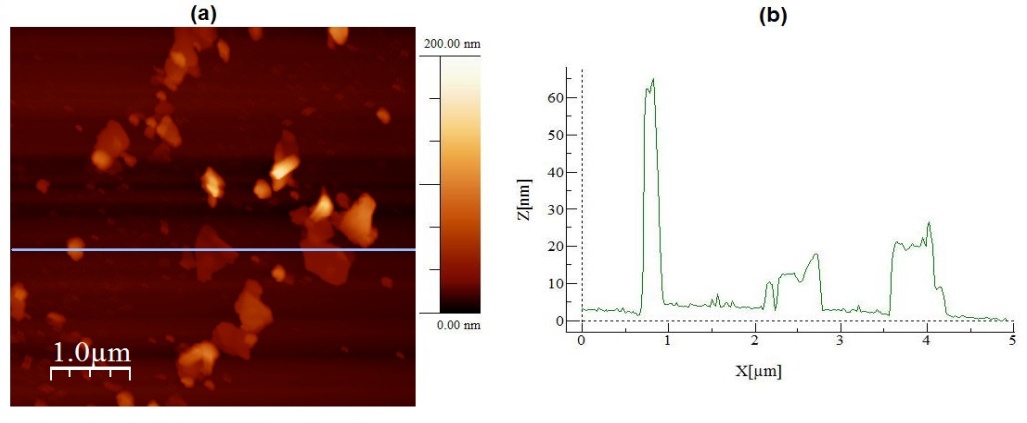
 | Figure 3. In (a) an AFM topographic image of a sample collected from the sonicated graphite and DMF solution for 7 hours. In (b) a topographic profile traced over the image presented in (a) |
|
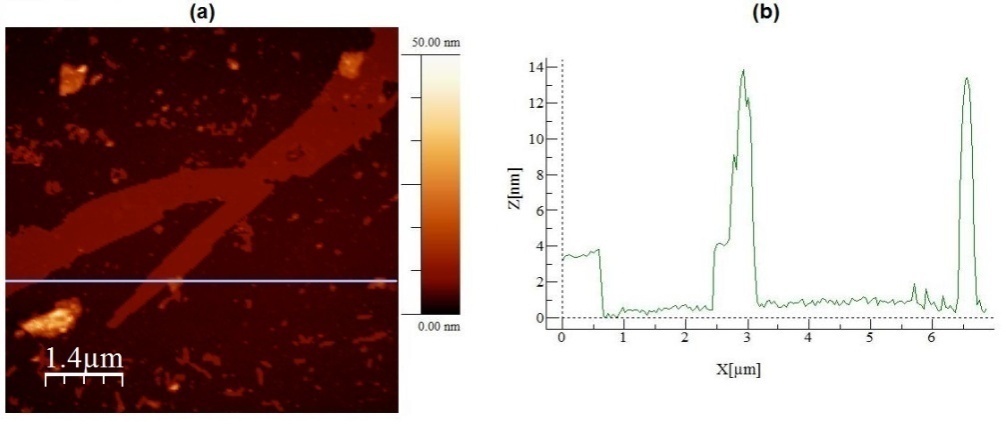
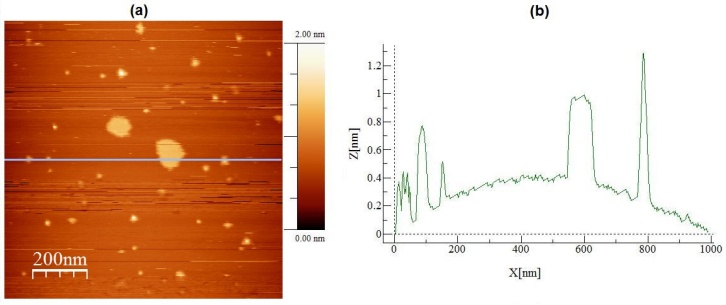 | Figure 4. In (a) AFM topographic image of graphite particles deposited onto mica surface. In (b) a topographic profile traced over the image presented in (a) |
4. Conclusions
- First, from the bibliographies studied in the present work, is possible to conclude that the ultrasonic bath technique is a powerful tool in obtaining graphite nanosheets and even graphene. Regarding the study of the effect of the sonication time interval on the dimensions of the graphite nanosheets, is possible to conclude that the longer the sonication time interval, the smaller the graphite nanosheets, with a decrease in at least one of the dimensions of the nanosheets with increasing the sonication time interval. Additionally, was possible to verify that with the Spin Coating technique is possible to obtain yet smaller graphite nanosheets, with thicknesses of 1 nanometer, similar to a graphene sheet.
ACKNOWLEDGEMENTS
- The heading of the Acknowledgment section and the References section must not be numbered.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML