-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2021; 10(1): 1-6
doi:10.5923/j.nn.20211001.01
Received: Jan. 15, 2021; Accepted: Feb. 2, 2021; Published: Feb. 26, 2021

Comparison Between the Biophysical Properties of Zinc Oxide in Both Its Bulk and Nanoforms
Rasha Said Shams Eldine, Ibrahim Y. Ibrahim
Medical Biophysics Department, Alexandria University, Alexandria, Egypt
Correspondence to: Rasha Said Shams Eldine, Medical Biophysics Department, Alexandria University, Alexandria, Egypt.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Despite the wide application of nanoparticles (NPs), there is a serious lack of information concerning their impact on human health and the environment. In fact, nano-materials are the leading edge of nanotechnology and their unique size-dependent properties make these materials superior and indispensable in many areas of human activity. Zinc oxide nanoparticles were produced synthetically by wet chemical route by using cheap precursors. The size of the particles were determined by X-ray Diffraction (XRD) and scanning electron microscopy (SEM) and was compared to that of bulk ZnO. structure and size Further studies were carried out to determine the difference in electrical properties (dielectric constant, dissipation factor, A.C. conductivity, resistivity) of these materials due to their size difference. Similar variations occurred in the liver enzymes; ALT, AST, as a result of treated the rats with ZnO in the bulk and Nano-forms. The increase in these enzymes indicated liver damage toxicity.
Keywords: Zinc oxide, XRD, SEM, Nanoparticles, Electrical properties
Cite this paper: Rasha Said Shams Eldine, Ibrahim Y. Ibrahim, Comparison Between the Biophysical Properties of Zinc Oxide in Both Its Bulk and Nanoforms, Nanoscience and Nanotechnology, Vol. 10 No. 1, 2021, pp. 1-6. doi: 10.5923/j.nn.20211001.01.
Article Outline
1. Introduction
- The production of nanoparticles, and nanostructures in general, has led to an increased interest in ZnO, taking into account the potential use in fields such as environmental remediation [1]. Zinc is an essential trace element widely exists in all body tissues, such as the brain, muscle, bone, skin, and so on. Also, zinc take parts in the metabolism of the body and plays critical functions in the synthesis of proteins and nucleic acid, neurogenesis, and hematopoiesis [2].Zinc oxide nanoparticles (ZnO-NPs) are selected as an antibacterial substance due to its superior characteristics, e.g., high specific surface area and the high activity to prevent a wide range of pathogenic factors. However, the antibacterial activity of ZnO NPs is still rarely known. Previous reports proposed the principal mechanisms of ZnO NPs antibacterial toxicity were based on the ability of these particles to induce excess ROS generation, such as superoxide anion, hydroxyl radicals, and hydrogen peroxide production [3]. The antibacterial activity may involve the accumulation of ZnO NPs in the outer membrane or cytoplasm of bacterial cells and trigger Zn2+ release, which would cause bacterial cell membrane disintegration, membrane protein damage, and genomic instability, resulting in the death of bacterial cells [4,5]. ZnO NPs are less expensive and of comparatively less toxic property offer superior biomedical applications, such as anticancer substance, drug delivery, antibacterial material, and diabetes curing, anti-inflammatory agent, wound healing; and bioimaging [6,7]. Biodistribution experiments have revealed liver, kidney, and spleen as the target organs for engineered nanoparticles after uptake by the gastrointestinal tract [8]. It has been reported that ZnO-NPs could ameliorate the behavioral and cognitive impairment in mice with depressive-like behaviors, probably through up-regulating neuronal synaptic plasticity and functions. Moreover, ZnO NPs may regulate ionic homeostasis and the physiological functions of neurons and have potential influence in central nervous system which shed light on the possible application and treatment in neurotransmitter system disorders [9].The aim of the present study is to study the biophysical properties of zinc oxide nanoparticles, and its toxicity in the liver tissues in both forms (nanoform and bulk form).
2. Materials and Methods
- The present study was carried out on 50 adult male albino rats weighing (100-150 g) were housed in polyethylene cages inside a well-ventilated room. Each cage contained 5 mice. Animals were maintained under standard laboratory conditions of temperature of 18-23°C with 40-60% humidity are recommended, and 12 hours light/dark cycle.The use of experimental animals in the present study was carried out in accordance with the Guiding principles for Biomedical Research Involving Animals (Appendix 2) of the Medical Research Institute, Alexandria University.Animals were randomly divided into the following groups, each of 10 rats, all the administered saline and /or bulk or nano ZnO were administered by gavage. • Group I: The animals of this group were orally administered with saline solution daily for 14 days, until the beginning of the experiment and served as a control group. • Group II: Orally administered with Zinc oxide NPs suspension (dissolved in saline) at concentration of 50 mg/kg, daily for 14 days.• Group III: Orally administered with Zinc oxide suspension (dissolved in saline) at concentration of 50 mg/kg, daily for 14 days.
2.1. Sampling
- • At the end of the experimental period (14 days), animals were anesthetized with ether and sacrificed by cervical dislocation. From each animal, blood was withdrawn directly from the heart. Part of the blood was collected in test tubes containing EDTA for complete blood picture (CBC).• Blood Serum:• The blood was centrifuged at 1400 rpm for 10 minutes. Serum was separated and stored at -4°C for determination of routine laboratory and biochemical parameters investigations.
2.2. Methods
- I Preparation of ZnO NPs [10]ZnO nanoparticles were prepared by the precipitation method. To the aqueous solution of zinc sulphate, sodium hydroxide solution was added slowly dropwise in a molar ratio of 1:2 under vigorous stirring, with the stirring was continued for 12 hours. The precipitate obtained was filtered and washed thoroughly with deionized water. Finally Zinc Oxide Nanoparticle (ZnO Nps) was prepared by calcining the precipitate at 500°C for 1 hour.II Characterization of prepared nanoparticles [11]1. Particle size analysis:The particle size distribution of the prepared particles was determined by laser light scattering on a Nano Zeta sizer particle analyzer (Malvern, UK), Central Lab, Faculty of Pharmacy, University of Alexandria, Figure (). The prepared particles were added to the sample dispersion unit containing the stirrer and stirred to reduce the aggregation between them, and laser obscuration range was maintained at 15–20%. The mean particle size was measured after performing the experiment in triplicate. Using cumulative analysis software and exponential sampling method, hydrodynamic size distribution.2. The shape of the particles:The physical size and shape of the prepared nanoparticles were determined by Scanning electron microscope (JEOL JSM-5300), Faculty of science.3. The absorption maxima:The absorption maxima at different wavelengths of visible light and UV were determined using UV-visible spectrophotometer.4. Electrical Analysis of both zinc sulphate and ZnO Nanoparticles.Electrical studies have been carried out in the frequency range of 20 Hz to 2MHz at room temperature for the two-particular material. Electrical properties such as dielectric constant (εr), dissipation factor (tan δ), A.C conductivity (σa.c.) and resistivity (ρ) of the samples were measured by using LCR meter (HP Model 4274 A, Hewlett-Packard, USA). For the measurement of electrical properties sample pellets of uniform thickness were prepared, and silver paste was coated on two extreme surfaces of the pallet to make those surfaces conducting.III Biochemical parameters• Liver enzymes activity in serum:• 1- Alanine aminotransferase (ALT) [12]• 2- Aspartate aminotransferase (AST) [12]
3. Results
- I Characterization of prepared ZnO nanoparticles as follow:1- Particle size analysis:
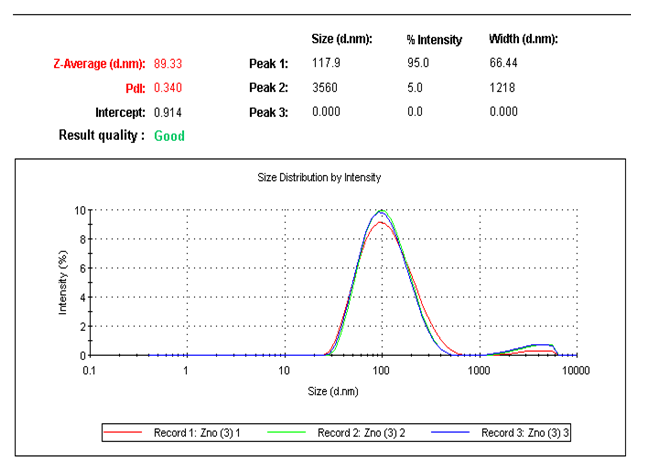 | Figure 1. The particle size distribution curve for ZnO nanoparticles |
 | Figure 2. SEM image for ZnO nanoparticles |
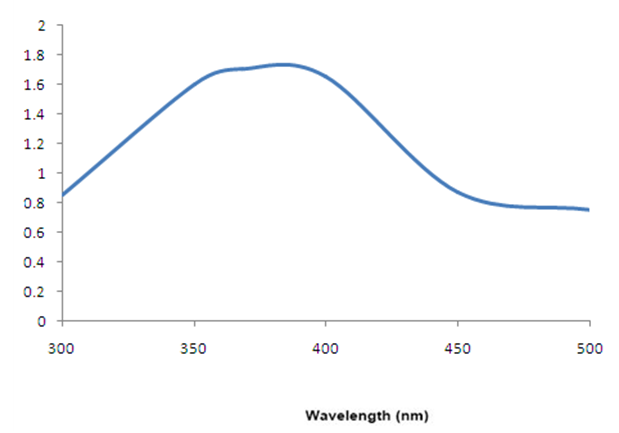 | Figure 3. Absorption spectra of ZnO nanoparticles |
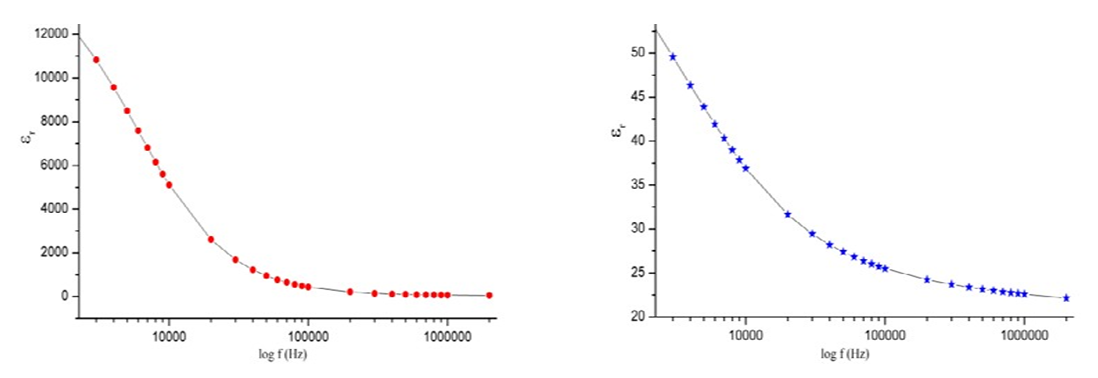 | Figure 4. The variation of the dielectric constant with respect to the logarithm of frequency |
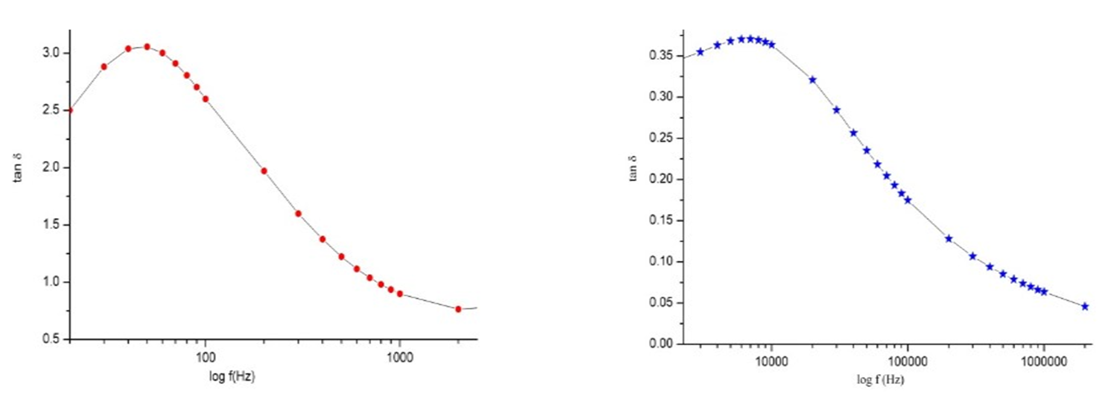 | Figure 5. The variation of the dielectric loss with respect to the logarithm of frequency |
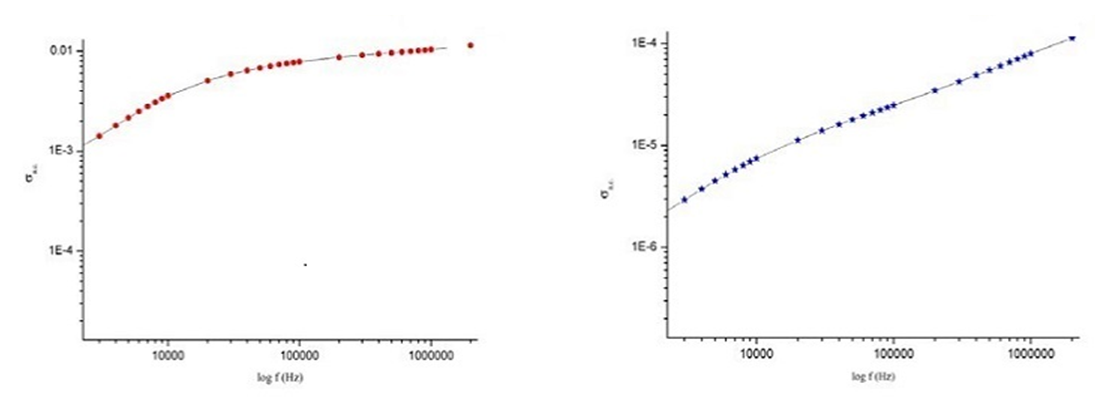 | Figure 6. The variation of the A.C conductivity with respect to the logarithm of frequency |
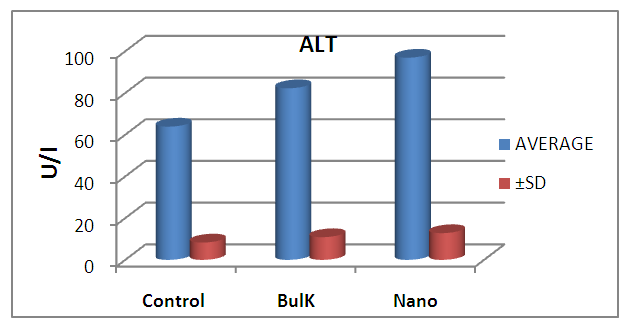 | Figure 7. Graphical illustration of ALT in blood of rats groups |
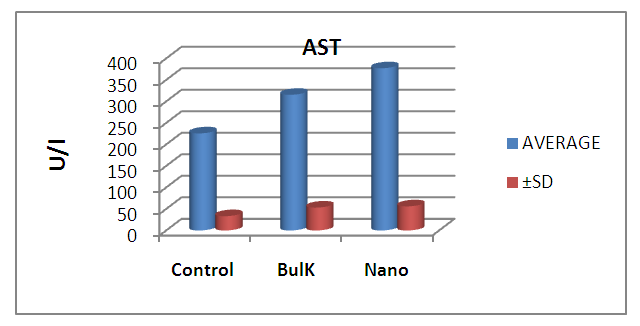 | Figure 8. Graphical illustration of AST in blood of rats groups |
4. Discussion
- The results suggest that the dielectric constant strongly depend on the frequency of the a.c. signal also on the different temperatures of the ZnS nanoparticles. The dielectric constant has higher values in the lower-frequency (20 Hz) due to the contribution of electronic, ionic, dipolar and space charge polarizations which has a strong dependence on frequencies [13] and then it decreases up to the high frequency (2 MHz). The electrode blocking layer is a dominated mechanism at lower frequency, thus the dielectric behavior is affected by electrodes polarization. At higher frequency, the dipole cannot rotate rapidly, so that, their oscillations lag those of the field. As the frequency is increased further the dipole will be completely unable to follow the field, and the orientation polarization ceases, so decrease approaching a constant value at higher frequencies due to interfacial polarization only [14].It can be seen that figure 1(a) has much higher value of єr compared to figure 1(b), due to the difference in size of synthesized ZnS material. ZnS nanoparticle is much conducting than ZnS bulk particle, so it helps to increase the value of dielectric constant as shown in figure 1(a). The results suggest that the dielectric constant strongly depend on the frequency of the AC signal also on the different temperatures of the ZnS nanoparticles. The dielectric constant has higher values in the lower-frequency (20 Hz) due to the contribution of electronic, ionic, dipolar and space charge polarizations which has a strong dependence on frequencies [13] and then it decreases up to the high frequency (2 MHz). The electrode blocking layer is a dominated mechanism at lower frequency, thus the dielectric behavior is affected by electrodes polarization. At higher frequency, the dipole cannot rotate rapidly, so that, their oscillations lag those of the field. As the frequency is increased further the dipole will be completely unable to follow the field, and the orientation polarization ceases, so decrease approaching a constant value at higher frequencies due to interfacial polarization only [14].Dielectric loss also shows a trend like the one shown by the dielectric constant. The decrease in the dielectric loss with the increase in frequency for all the temperatures suggests that the dielectric loss is strongly dependent on the frequency of the applied field. The high values of dielectric loss at low frequencies could be related to the charge lattice defect of the space charge polarization [19]. Dielectric loss also shows a trend like the one shown by the dielectric constant. The decrease in the dielectric loss with the increase in frequency for all the temperatures suggests that the dielectric loss is strongly dependent on the frequency of the applied field. The high values of dielectric loss at low frequencies could be related to the charge lattice defect of the space charge polarization [13].In low signal frequency range, when the electrical dipoles are an able to follow the variation of the electric field, the dissipation factor decreases with the increases of signal frequency [15]. DF generally varies depending on the dielectric material and the frequency of the electrical signals. In low dielectric constant (low-k), temperature compensating ceramics, DF is low, whereas in high dielectric constant ceramics, the value of DF is higher. However, in our study the same thing is observed. As, ZnS nanoparticle gives rises to high-k, its dissipation factor value is 7% higher than that of the bulk one.Also AC. conductivity has weak temperature dependence and dominates at higher frequency and low temperature and D.C conductivity depends strong on temperature and dominates at lower frequency and high temperature.Similar variations occurred in the liver enzymes; ALT, AST, in the treated rats with Zn in the bulk and Nano-forms. The increase in these enzymes indicated liver damage toxicity, which may be accompanied with disturbance in the production of the clotting factors, albumin, and many other important proteins synthesized by liver.However, according to Fazilati (2013) [16], the results showed that activity of ALT, AST enzymes increased in the treated group with ZnO NPs compared to the control group.
5. Conclusions
- ZnO NPs has more toxic effect than the bulk from of ZnO.
Appendix
- Similar variations occurred in the liver enzymes; ALT, AST, and ALP as a result of treated the rats with Zn in the bulk and Nano-forms, with no effect on bilirubin. The increase in these enzymes indicated liver damage toxicity, which may be accompanied with disturbance in the production of the clotting factors, albumin, and many other important proteins synthesized by liver.However, according to Fazilati (2013), the results showed that activity of ALT, AST and ALP enzymes Increased compared to the control group in group that received 200ppm ZnO Nanoparticles.II Comparison between the effect of ZnO in the bulk form and nano particles on the following:A The liver enzymes activity and the related biochemical analysis
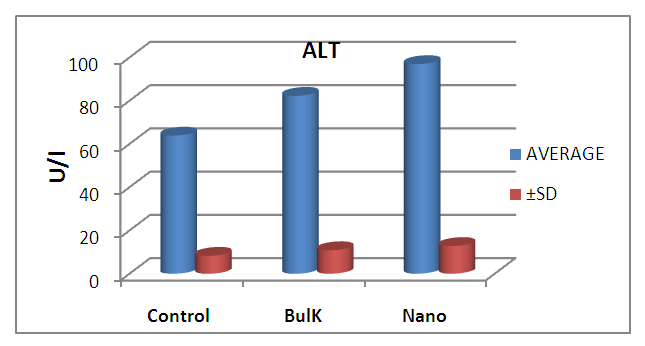 | Figure 9. Graphical illustration of ALT in blood of rats groups |
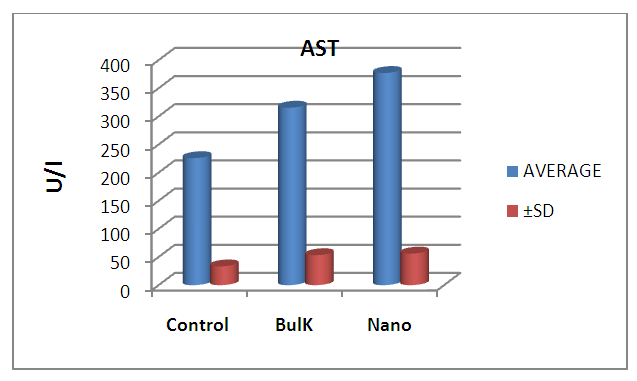 | Figure 10. Graphical illustration of AST in blood of rats groups |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML