-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2019; 9(2): 37-43
doi:10.5923/j.nn.20190902.01

Preparation of Coal-Kaolinite Nano Composites and Investigation of Their Use to Remove Methyl Orange from Water
Misael Silas Nadiye-Tabbiruka , Fortunate Phenyo Sejie
Department of Chemistry, University of Botswana, Gaborone
Correspondence to: Misael Silas Nadiye-Tabbiruka , Department of Chemistry, University of Botswana, Gaborone.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Coal/clay nano composites of ratios 1:1, 1:4, 4:1 were successfully prepared and characterised by electron microscopy. They were then used to study kinetics and thermodynamics of removal from water of methyl orange of various concentrations, various pH and at various temperatures. The coal:clay ratios 1:1 and 4:1 performed equally but better than 1:4 and the individual coal and clay samples. The swelling in coal and in clay which occurs on initial adsorption appears to have been eliminated by the miniaturisation. The adsorption capacity of the composites was found to be higher than that of either the clay or the coal on their own but it is still lower than that of commercial activated carbon.
Keywords: Adsorption, Nano composite, Adsorption kinetics, Adsorption thermodynamics, Coal, Kaolinite
Cite this paper: Misael Silas Nadiye-Tabbiruka , Fortunate Phenyo Sejie , Preparation of Coal-Kaolinite Nano Composites and Investigation of Their Use to Remove Methyl Orange from Water, Nanoscience and Nanotechnology, Vol. 9 No. 2, 2019, pp. 37-43. doi: 10.5923/j.nn.20190902.01.
Article Outline
1. Introduction
- Nano particles, colloid materials with average size range of 1-100nm, are very useful for fundamental and diversified applications [1]. They are used in several industrial applications including optical sensor devices, thermal conducting gel, body repairing, cancer therapy, high performance batteries, chromatography columns and magnetic fluids to mention but a few. In pure form they cannot be used because of their tendency to agglomerate. To avoid these problems, nano particles are embedded on a support material to form nano composites instead [2]. Preparation methods for nano composite particles depend on their intended use. Two most widely used methods for preparing nanoparticle/composite are chemical and physical procedures [3]. Chemical synthesis involves the use of chemicals reactions to produce nano units while physical methods involve breaking down of large material by mechanical grinding until the particles are in the nano range [4]. Furthermore, coal and Clay minerals are widely used in several industries and fields such as in polymers nano-composites adsorbents fields, in heavy metal ion adsorption, ceramics, and paper fillings [5-7] as well as in nano composites designed for specific applications. These include anion adsorbents [8,9], cation adsorbents [10,11,8] and in some cases adsorbents to aid purification of water by removing multiple contaminants simultaneously [12].In this paper we present the preparation and characterisation of coal/clay nano composites. These composites are then used to investigate their possible use to purify water. This is done by studying the adsorption kinetics and thermodynamics of removal from water of methyl orange, an organic pollutant.
1.1. Adsorption Kinetics
1.1.1. Lagergren Pseudo 1st Order Kinetics’Model
- Lagergren pseudo first-order rate equation, the earliest known model describing the adsorption rate based on the adsorption capacity [9], is given below (equation 1):
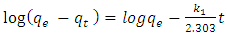 | (1) |
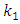 is the pseudo first order rate constant in g/mg* min, qe is the amount adsorbed at equilibrium, qt is the amount adsorbed at any time t, in minutes. If the data obtained fits the model, a plot of
is the pseudo first order rate constant in g/mg* min, qe is the amount adsorbed at equilibrium, qt is the amount adsorbed at any time t, in minutes. If the data obtained fits the model, a plot of 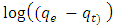 against t should give a straight line and
against t should give a straight line and 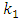 can be determined from the slope and
can be determined from the slope and 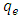 from the intercept. The rate constant the theoretical and the equilibrium amount adsorbed qe can be derived from this model plot.
from the intercept. The rate constant the theoretical and the equilibrium amount adsorbed qe can be derived from this model plot.1.1.2. The Lagergren Pseudo Second Order Model is Given by Equation 2
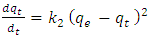 | (2) |
1.2. Adsorption Thermodynamics
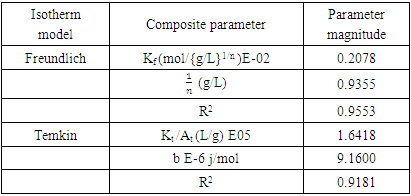
- Adsorption thermodynamics’ data is analysed using Langmuir, Freundlich and Temkin models, in this work the data did not fit the Langmuir isotherm hence it was not used. The Freundlich adsorption model is given by equation 3 and 4
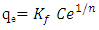 | (3) |
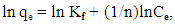 | (4) |
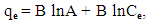 | (5) |
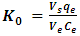 | (6) |
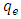 is the surface concentration of dye on adsorbent (mol g-1)
is the surface concentration of dye on adsorbent (mol g-1) 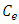 is the concentration of dye in the equilibrium solution (mol L-1),
is the concentration of dye in the equilibrium solution (mol L-1), 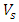 is the activity coefficient of the adsorbed dye and
is the activity coefficient of the adsorbed dye and 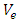 is the activity coefficient of dye in solutionFor very dilute solutions
is the activity coefficient of dye in solutionFor very dilute solutions 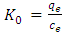 hence the values of Ko is obtained from a plot of
hence the values of Ko is obtained from a plot of 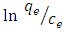 versus qe. An extrapolation of qe to zero [13] gives an intercept equal to ln Ko. Thus the thermodynamics parameters can be calculated from equation 7 and 8 given below.
versus qe. An extrapolation of qe to zero [13] gives an intercept equal to ln Ko. Thus the thermodynamics parameters can be calculated from equation 7 and 8 given below.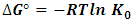 | (7) |
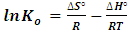 | (8) |
2. Experimental
2.1. Materials
2.1.1. Adsorbent
- The coal-clay nano-composites were prepared using a method adopted from the literature [14,15] in which nano- composites with activated interfaces were prepared by mechanical grinding of magnesium hydrides used for storing hydrogen.Mixtures of 1:1, 1:4 and 4:1 mass ratio of the coal (ESEM fig 1a) to clay (ESEM fig 1b [16]) were made and used to prepare the composites. The resulting powder was then soaked in deionised water at room temperature under ultrasonic stirring, to form a homogeneous mixture. The resulting mixture was subjected to mechanical grinding until the slurry mixture was formed in water [17]. The resulting mixture was centrifuged at 2000 rpm. Water was decanted and the top fine particles were dried in an oven. The resulting product was analysed with an Environmental Scanning Electron Microscope (ESEM figure 1c). This was repeated until particle reduction to nano level was achieved. These were used to investigate adsorption experiments.
 | Figure 1a. ESEM microgra of Morupule coal |
 | Figure 1b. ESEM microgram of Lobatse clay [16] |
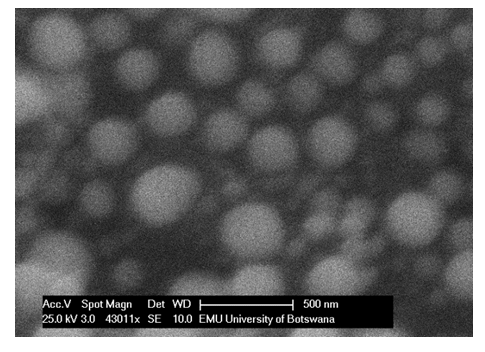 | Figure 1c. SEM microgram of a 1:1 clay: coal composites |
2.1.2. Adsorbate Methyl Orange (MO)
- Methyl orange (Anionic, water soluble azo dye) powder (C14H14N3NaO3S, figure 2a) supplied by SAARCHEM, South Africa was used without any further purification. A solution of the dye was made by dissolving a known weight into double distilled de-ionised water. Other required dye concentrations were prepared by serial dilution. Several of these concentrations were used to obtain a calibration curve of absorbance against concentration at a predetermined wavelength of maximum absorption λ=463 nm using a UV-Visible spectrophotometer spectronic 20. Concentrations of unknown samples were then determined from the calibration curve.
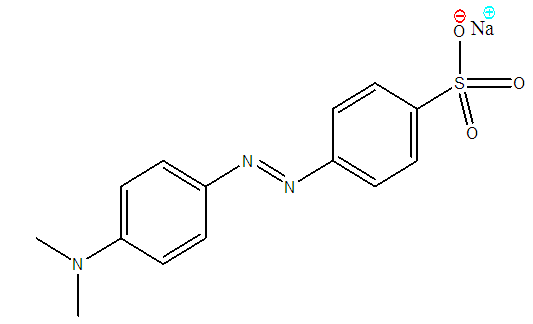 | Figure 2. Methyl orange structure |
2.2. Procedure
2.2.1. Studies of Adsorption Kinetics
- 0.05g of the nanocomposite was added to 20 ml of MO solution in a screw capped bottle contained in a water bath at constant temperature, and a stop clock was simultaneously started. Samples of the supernatant liquid were drawn off after predetermined time intervals, centrifuged at 2000 revolutions per minute for a minute or so to assist the separation of the nano particles from the supernatant, filtered using an appropriate filter paper and then their absorbance were taken using a UV/VIS spectrophotometer at a wavelength of 463 nm. The amount adsorbed was obtained by difference between the initial concentrations and the concentrations at the sampling time and plotted against time. The experiment was repeated for various MO concentrations, reaction temperature and pH.
2.2.2. Studies of Adsorption Thermodynamics
- In this section, a batch technique was used. The clay-coal composite (0.05 g) was added to each of several 250 mL screw capped bottles containing 200ml of methyl orange of different initial concentrations. The bottles were kept in a thermostatic water bath shaker at a speed of 220 rpm for 4 hours at constant temperature. After the samples had reached equilibrium, the Solutions were centrifuged, filtered using 0.45 μm filter paper and the equilibrium concentrations of the filtrates were determined spectrophotometrically as before. The amount adsorbed at equilibrium, qe (mol/g), was calculated by using equation 9 below:
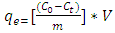 | (9) |
3. Results and Discussion
3.1. Analysis of Results from Adsorption Kinetics Studies
- Figure 3a presents the adsorption kinetics of the dye onto the composites at 300K. For the three composites, the process reaches equilibrium within 30minutes. The composites show different adsorption capacities and different rates of adsorption at the various ratios. The nano composite with the highest coal content removes more of the dye compared to the other two. The nano composite with highest mass of coal and that with equivalent masses of coal and clay show the highest adsorption capacity and rate. The nano composite with the lowest coal content shows lowest adsorption capacity.
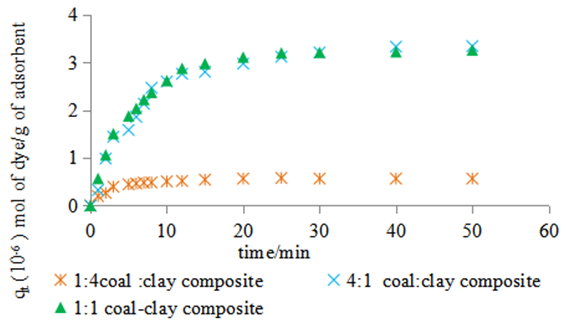 | Figure 3a. Adsorption of methyl orange dye 3.14 × 10-5 M on to the composites at pH 7.18 and 300K |
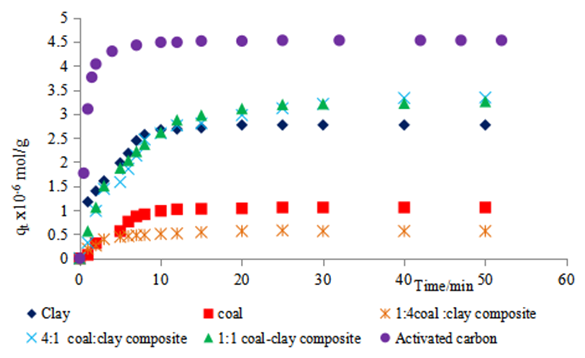 | Figure 3b. Studies of methyl orange 3.14 x 10-5 M adsorption onto various adsorbents at 300K |
3.1.1. Testing data on Kinetic Models
- The chi (χ2) test was used to test the closeness of the calculated/theoretical data to the experimental data, by using equation 10.
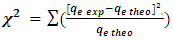 | (10) |
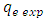 is the experimentally determined equilibrium amount adsorbed
is the experimentally determined equilibrium amount adsorbed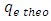 is the theoretical equilibrium amount adsorbed determined from the kinetics’ model equationsFrom this equation a small value of
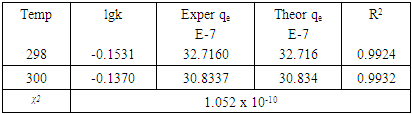
is the theoretical equilibrium amount adsorbed determined from the kinetics’ model equationsFrom this equation a small value of 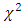 shows the model test fits the experimental data well.Adsorption of methyl orange onto Morupule coal:Lobatse clay nano composite was investigated by fitting the raw data to the lagergren pseudo first and pseudo second order kinetic models and the resulting plots are given below (see fig 4, table 1).
shows the model test fits the experimental data well.Adsorption of methyl orange onto Morupule coal:Lobatse clay nano composite was investigated by fitting the raw data to the lagergren pseudo first and pseudo second order kinetic models and the resulting plots are given below (see fig 4, table 1). 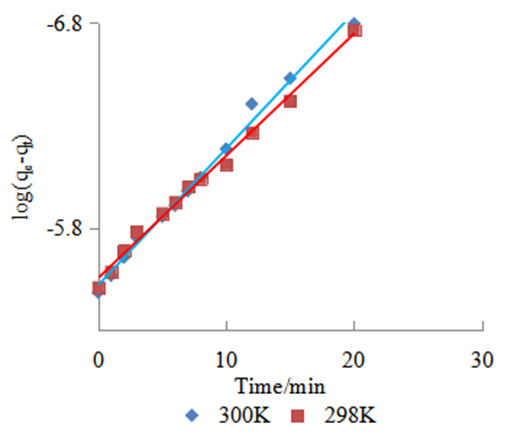 | Figure 4. Lagergren pseudo first order model plot for the adsorption of methyl orange onto the 1:1 composite at T= (298 and 300) K, 5.49 × 10-5 M, pH 7.18 and 10 g/L sorbent dosage |
|
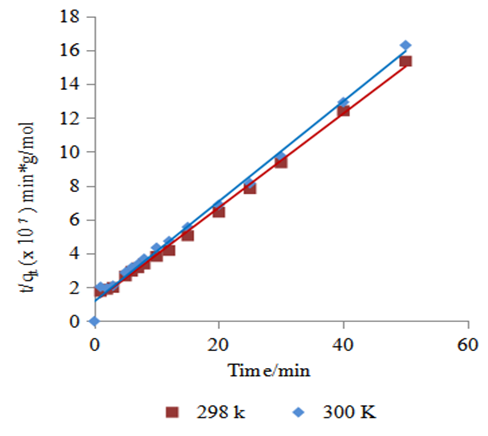 | Figure 5. Lagergren pseudo second order kinetics model for the adsorption of methyl orange onto the 1:1 coal: clay composite at T= (298 and 305) K, 5.49 × 10-5 M, pH 7.18 and 10 g/L sorbent dosage |
3.2. Thermodynamics’ Data Analysis
- Figure 6 shows a decrease in the adsorption capacity of the nano composites with an increase in pH indicating that adsorption is more favoured in acid conditions than in basic conditions.
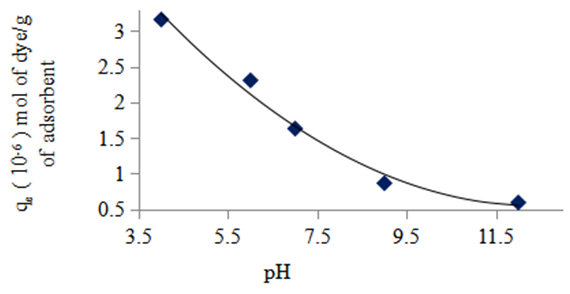 | Figure 6. Effect of pH change on the equilibrium adsorption of methyl orange onto the 1:1 composite at 3.14 × 10-5 M, 298 K and 10 g/L sorbent dosage |
3.2.1. Effect of pH on the Structure of the Dye
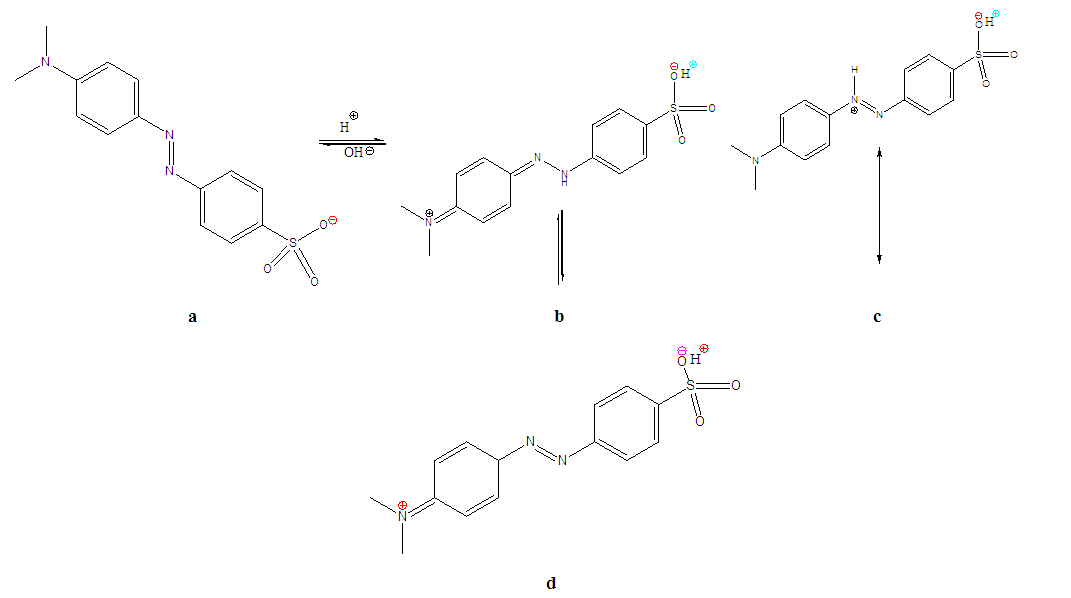 | Figure 7. Alkaline form of Methyl orange (a) and its mono-protonated zwitter ionic structures under acidic conditions(b, c and d) |
3.2.2. Temperature Effect
|
|
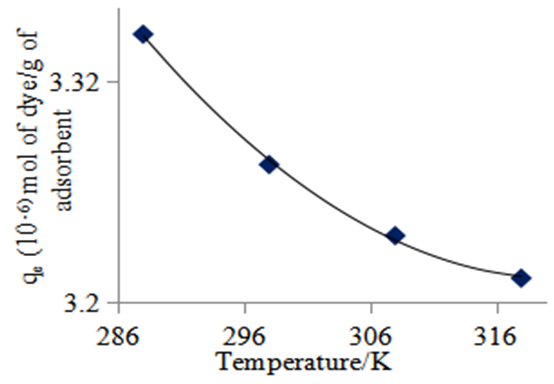 | Figure 8. Effect of change in temperature on the equilibrium adsorption of methyl orange onto the 1:1 composite at 3.14 × 10-5 M, 10 g/L sorbent dosage and pH 7 |
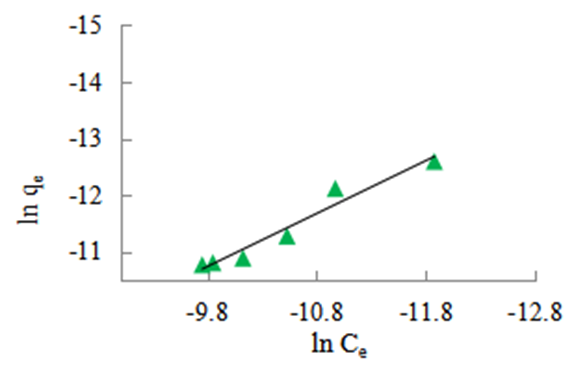 | Figure 9. Freundlich isotherm for the adsorption of methyl orange onto 1:1 nano composite at 298 K, pH 7.18 and 10 g/L sorbent dosage |
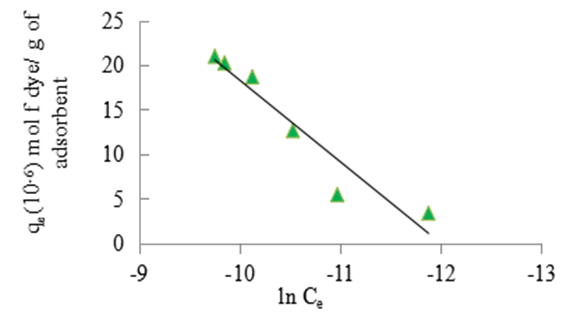 | Figure 10. Temkin isotherm for the adsorption of methyl orange onto the 1:1 coal-clay composite at 298 K, pH 7.18 and 10 g/L sorbent dosage |
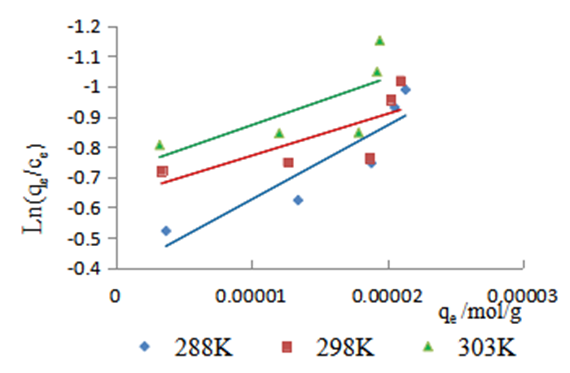 | Figure 11. Plot for the determination of Ko at various temperatures for the adsorption of methyl orange onto the 1:1 composite |
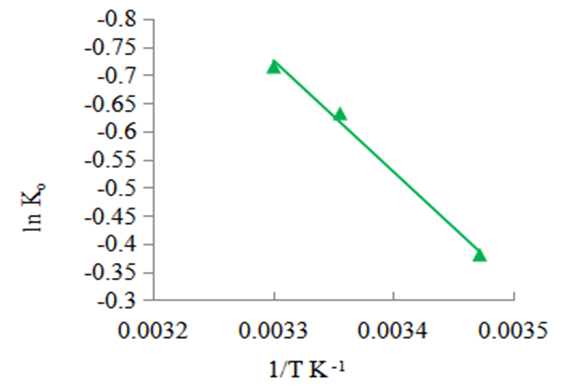 | Figure 12. Vant’Hoff plot for the adsorption of methyl orange onto the 1:1 nano composite |
4. Conclusions
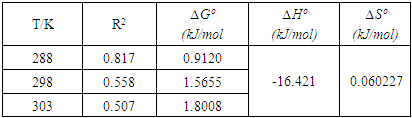
- The possible use of the composites as water purifiers investigated by studying their interaction thermodynamics and kinetics at various temperatures and solution pH found that adsorption capacity, qe, increases with increasing initial concentration (see fig 9), decreases with increasing pH (see fig 6) and decreases with increasing temperature (see fig 8). The enthalpies and entropies of adsorption were found to be -16.421 kJmol-1 and 0.060 kJmol-1 respectively (see table 3) for the 1:1 composite indicating that physisorption is still the mechanism in operation. It is evident that miniaturisation to nano level greatly improves their capacity and rate for adsorbing methyl orange compared to that of the individual clay or coal samples. The fitting of the kinetics data on Lagergren pseudo second order was better than that on the pseudo first order. However the fitting on pseudo first order greatly improved from the cases of the individual coal and clay adsorbents observed in earlier work [21]. This indicates that as a result of miniaturisation, the internal areas originally accessed after swelling caused by initial adsorption which often lead to a second adsorption surge was now easily available at the start. There was therefore no adsorption initiated expansion of the material which was observed for both the clay and the coal individually [21,22]. However, its contribution to the overall available area is less that the contribution from steam activation since the commercial steam activated carbon still performed better indicating that steam activation is more effective in creating a super adsorbent than miniaturisation. This is therefore a cheap viable method of removing methyl orange comparable to many others [23-26].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML