-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2019; 9(1): 1-21
doi:10.5923/j.nn.20190901.01

Synthesis of Triazine-Based Dendrimer Assisted Pd-Cu Bimetallic Nanoparticles and Catalytic Activity for C-C Coupling Reactions
Md. Sayedul Islam, Md. Wahab Khan
Department of Chemistry, Faculty of Engineering, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh
Correspondence to: Md. Sayedul Islam, Department of Chemistry, Faculty of Engineering, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Triazine-based dendrimer assisted Pd-Cu bimetallic nanoparticles (NPs) were synthesized through the sequential loading method from the dendrimer which was prepared by the reaction of 2,4,6-triamino-1,3,5-triazine with benzoyl chloride in DMF at 80°C at 8 hrs. The dendrimer was characterized by IR, NMR, and elemental analysis. SEM and EDX of Pd-Cu bimetallic NPs demonstrated nanosized spherical surface morphology as well as the existence of palladium ions, and copper ions in the nanoparticles whereas TGA and DSC confirmed good thermal stability of the nanoparticles, in addition, XRD data and the analysis of TEM revealed the average 18.50 nm nano shape FCC structure of the particles. These NPs were found to be the effective heterogeneous a catalyst for the C-C cross-coupling reactions such as Heck, Sonogashira in excellent yields. The high stability, reusability, and heterogeneity of recovered catalyst was also observed by further analysis (SEM, EDX, XRD, TEM and leaching study). 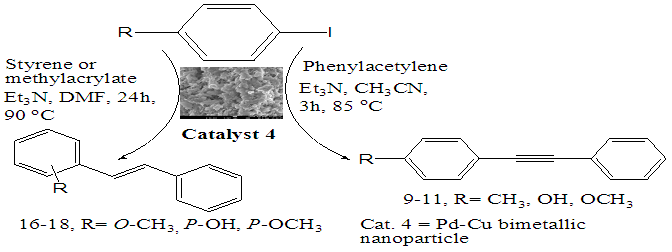
Keywords: Bimetallic nanoparticles (NPs), Triazine, Dendrimer, phenylacetylene, Heck and Sonogashira reaction
Cite this paper: Md. Sayedul Islam, Md. Wahab Khan, Synthesis of Triazine-Based Dendrimer Assisted Pd-Cu Bimetallic Nanoparticles and Catalytic Activity for C-C Coupling Reactions, Nanoscience and Nanotechnology, Vol. 9 No. 1, 2019, pp. 1-21. doi: 10.5923/j.nn.20190901.01.
Article Outline
1. Introduction
- Bimetallic nanoparticles, composed of two different metals, often display stepped forward catalytic performances and applications in numerous industrial processes, on the whole in fuel industries or environmental catalytic approaches, and more recently in C–C cross-coupling reactions [1]. Bimetallic catalysts constitute an interesting magnificence of catalysts due to the fact one metal can tune and/or alter the catalytic properties of the alternative because of the electronic and structural interactions [2]. On the other hand, several metal-catalyzed cross-coupling reactions require the presence of another metal either as a co-catalyst or to help manipulate the general manner such as Sonogashira reaction among an aryl halide and terminal alkynes calls for an aggregate of Pd and Cu as the catalysts, in which Cu+ plays a catalytic role in shifting the alkynyl groups to Pd [3]. The splendid redox properties of the Pd/Cu method are due to the electron donor and acceptor individual of Cu and Pd, respectively [4,5]. The prominent “Cu-effect” within the Pd-catalyzed cross-coupling reaction [6,7] has enhanced the improvement of several heterogeneous bimetallic NPs the use of commercially to be had insoluble polymeric help and has been used efficaciously in various cross-coupling reactions [8-14]. The main disadvantages of the Sonogashira reaction has been idea-about to rise up from the high price of palladium catalyst and its low reusability, the residual contamination of a poisonous palladium species (the main downside in the synthesis of pharmaceuticals), and consequently the utilization of copper salt sensitive to oxygen that elicited a side reaction, i.e., oxidative homocoupling of the alkyne substrates (the so-referred to as Glaser-coupling reaction) [15-17]. In addition, many improved protocols for Suzuki–Miyaura [18-20] and Heck [21] couplings have been reported with the use of bimetallic Pd-Cu NPs. Our research has been centered at the event of an additional green, extra price, and additional simply formulated catalyst which can be active enough to perform the carbon-carbon coupling reaction under moderate conditions.Furthermore, Bimetallic DENs used as catalysts have discovered numerous crucial applications, and research pursuits have already progressed far beyond the improvement of the synthetic method to synthesize the designed dendrimer templated nanoparticles [22]. Because of the synthesis of DENs with the dendrimer as the template has the following benefits: (1) the products have for the most component an undistinguishable composition and shape since the dendrimers template themselves and construct properly-defined nanoparticle replicas; (2) the probabilities for the nanoparticles to agglomerate during the catalytic reactions are declined substantially as they're stabilized and encapsulated within the dendrimers; (3) the impact of the catalytic active nanoparticles in the reactions may be aided considering the fact that the most portions at the surface of the nanoparticles are unpassivated. That is due to the fact the combination of nanoparticles in the dendrimers is specially induced by means of steric outcomes; (4) the branches of the dendrimer will play a characteristic of being a selective gate to control the entrance of the substrate to the catalytic nanoparticles inside the dendrimers; and (5) the periphery functional portions of the dendrimer can be tailored to adjust the miscibility of the DENS with the continuous phase and in addition to help in connecting to the surfaces and different polymers [23-37].However, to the great of our knowledge, there's less report to be had inside the literature for the synthesis of bimetallic NPs impregnated on triazine-based dendrimer and the exploration of their heterogeneous catalytic interest in cross-coupling reactions. Here we record the synthesis and characterization of a new elegance of heterogeneous Pd/Cu bimetallic dendrimer assisted that have proven efficient catalytic activity for the Heck and the Sonogashira cross-coupling reaction without using CuI as co-catalyst under phosphine-ligand-free conditions with high recyclability.
2. Materials and Methods
2.1. Materials
- All reactions involving air- and moisture-sensitive conditions were carried out in a dry nitrogen atmosphere. Unless otherwise noted, all reagents were reagent grade, and were used without purification. Dehydrated DMF, DMSO, CH3CN and THF were used as reaction solvent. These solvents were purchased from Aldrich and used as received. De-ionized water was used in the experiment where required. Analytical thin layer chromatography (TLC) was silica gel 60 F 254 coated on 25 TCC aluminum sheets (20 × 20 cm). Silica gel column chromatography separations were performed on silica gel 60 N (neutral, 40-100 μM). 2, 4, 6-triamino-1, 3, 5-triazine, benzoyl chloride and CuCl2.nH2O, H2N-NH2.H2O, PdCl2 were purchased from Aldrich and were directly used without further purification. K2CO3, NaOH, Cs2CO3, KOH, Na2CO3, Et3N, KtOBu (Sigma-Aldrich), and MgSO4 (Sigma-Aldrich) and also in the coupling reactions, styrene, methyl acrylate phenylacetylene were used (Sigma-Aldrich).
2.2. Instrumentations
- Melting points were determined by open capillary tubes by a melting point apparatus (Model BUCHI, B-540). The IR spectra were taken on a Shimadzu FTIR 8400S Fourier Transform. Infrared Spectrophotometer (400-4000 cm-1) with KBr pellets. 1H NMR and 13C NMR spectra were recorded at 500 MHz and 100 MHz, respectively, on a JEOL, JNM-ECZ 500 MHz instrument and also 1H NMR (400 MHz, Bruker) and 13C NMR (100 MHz, Bruker). Chemical shifts were given relative to TMS. Mass spectra (MS) were measured by using AXIMA-CFR, Shimadzu/Kratos TOF Mass spectrometer. Elemental analyses were carried out with a Fisons EA 1108 CHNS-O apparatus. Analytical thin layer chromatography (TLC) was silica gel 60 F 254 coated on 25 TCC aluminum sheets (20 × 20 cm). Silica gel column chromatography separations were performed on silica gel 60 N (neutral, 40-100 μM). The thermal behavior of Pd/Cu NP was determined by a thermo gravimetric analyzer (NETZSCH STA 449F3) from 26°C to 600°C. TG and DSC data were obtained under a nitrogen atmosphere by using aluminum oxide crucible at a heating rate of 10 k/min and at a flow rate of 40 and 60 ml/min. SEM and EDX of Pd/Cu bimetallodendrimer NPs 4 were taken by the JEOL-JSM-7600F. Particle size and phase identification were performed with a PANANALYTICAL x-ray diffractometer. TEM analysis was performed by Philips CM12 transmission electron microscope (operating at 200 KeV). The leaching study was done by Perkin Elmer ELAN DRC E ICP/MS.
2.3. Synthesis
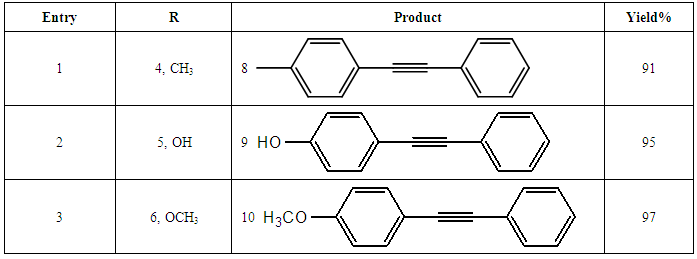
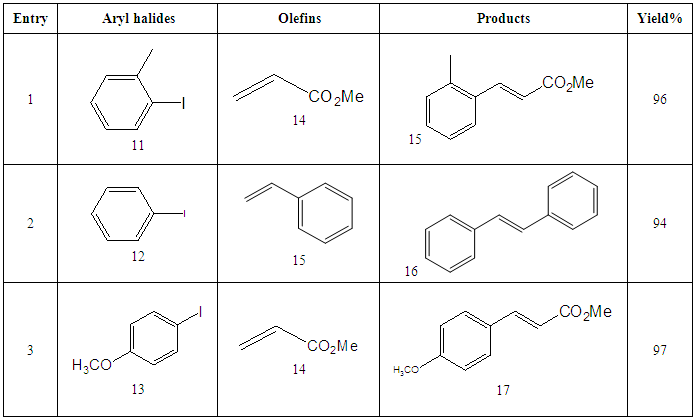
- Synthesis of 2, 4, 6-Tris (di-benzamido)-1, 3, 5-Triazine 3Benzoyl chloride 2 (1.45 g, 9.54 mmol) was in turn added to a solution of 2, 4, 6-triamino-1, 3, 5-Triazine 1 (0.2 g, 1.59 mmol) in DMF (10 ml). The solution was degassed and stirred for 8 hrs at 80 oC under a nitrogen atmosphere. The advancement of the reaction was checked by TLC. At the start of the reaction, the reactant mixture was a clear solution and progressively it was transformed into white solid. When the reaction was completed, distilled water was added and the white solid product was separated, washed with sodium bicarbonate solution by using Buchner funnel in addition as finally refined by re-crystallization with ethyl alcohol and was obtained the preferred product 3.White crystalline solid, m. p. 195-197 oC, odorless and 95% of yield. IR (KBr): δ max 3052.19, 2976.31, 1712.20, 1615.69, 1415.69, 1360.20 cm-1. 1H NMR (500 MHz, CDCl3): δ 7.45 (t, 12H, Ar-H, J =7.5 Hz), 7.62 (t, 6H, Ar-H, J=6.5 Hz), 8.15 (d, 12H, Ar-H, J=7.0 Hz).13C NMR (100 MHz, CDCl3): δ 128.60, 129.46, 130.34, 133.94, 144.75 and 172.77 ppm. Anal. Calcd. (%) for C45N6H30O6: C, 71.99; H, 4.03; N, 11.19. Found: C, 71.90; H, 4.00; N, 11.10.Synthesis of dendrimer templated Pd-Cu bimetallic nanoparticles 4The effective development of a diversity of the synthetic techniques for the preparation of bimetallic dendrimer nanocatalysts is key advances in recent years. Among the various methods, mostly three routes of preparing the bimetallic dendrimer nanocatalysts are commonly used which includes co-complexing, partial displacement, and sequential reduction [38]. Here we followed the sequential reduction method to synthesize Cu/Pd nanoparticles. The dendrimer of 0.113 g 2, 4, 6-Tris (di-benzamido)-1, 3, 5-Triazine 3 (0.15 mmol), 0.00095 g PdCl2, NH2-NH2.H2O (1.5 mmol) was stirred in CH3CN (10 mL) in a round bottom flask at 85°C for 1 h. Then 0.025 g CuCl2.2H2O (Pd-Cu weight ratio of 1:26) and NH2-NH2.H2O were added to the reaction mixture dropwise with a little more than the equivalent amount of copper salt. The NaOH solution (0.3 M) was slowly added to the reaction mixture to maintain pH 10 and the suspension was stirred vigorously for 2.5 hours using a magnetic stirring bar at 100°C. After settling the reaction mixture, the black residue was obtained. The residue was filtered after centrifugation (4000 r.p.m. for 10 min) of the reaction mixture, washed with double distilled water and acetone and dried overnight in an oven at 130°C.Catalytic performance of Pd-Cu bimetallic nanoparticle 4 for Sonogashira ReactionGeneral method: A Round Bottom flask was charged with 1.0 mmol of aryl iodide, 1.2 mmol of phenylacetylene, 1.5 mol % of Pd-Cu bimetallic NP 4, Et3N (2 mL) as a base and CH3CN (5 mL) as a solvent under the nitrogen atmosphere. The mixture was then stirred at 85°C for 3 hours, and the reaction was checked by TLC. After the reaction was completed, the mixture was extracted with CHCl3 (15 mL). The organic layer dried with MgSO4 was separated, filtered and concentrated to supply crude product purified by silica gel column chromatography using ethyl acetate and hexane (6:1) to produce preferred pure products under reduced pressure.Synthesis of 1-(2-p-tolylethynyl)benzene 9, [38]Solid colorless product was obtained, yield: 91%, m.p. 71-73°C (lit. 71°C); 1H NMR (400 MHz): δ 2.32(s, 3 H); 7.21 (d, 2H, J= 8.8 Hz); 7.33 (t, 3H, J= 5.6 Hz); 7.34 (d, 2H, J=8.4 Hz); 7.55 (d, 2H, J= 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ 92.52, 93.14, 120.21, 122.24, 128.28, 128.49, 128.98, 132.04, 133.94, 139.14.Synthesis of 4-(2-phenylethynyl)phenol 10, [38]Solid product was obtained, yield: 95%, m.p. 124-126°C; 1H NMR (CDCl3, 400 MHz): δ 5.07 (s, 1 H, OH); 6.74 (d, 2H, J=8.8 Hz); 7.28-7.36 (m, 3 H); 7.44 (d, J= 8.8, 2H); 7.54 (d, J=8.8, 2H). 13C NMR (100 MHz, CDCl3): δ 93.92, 114.14, 115.31, 123.98, 128.48, 129.04, 133.94, 134.44, 159.52.Synthesis of 1-(2-(4-methoxyphenyl)ethynyl)benzene 11Solid white product was obtained, yield: 97 %, m.p. 57-59°C (lit. 57°C); 1H NMR (400 MHz): δ 3.72 (s, 3H); 6.89 (d, 2H, J=8.8 Hz); 7.34 (t, J=10.8 Hz, 3H); 7.35 (d, 2 H, J=8.8 Hz); 7.45 (d, 2H, J=8.0 Hz), 13C NMR (100 MHz, CDCl3): δ 55.52, 93.24, 113.24, 115.21, 123.38, 128.49, 129.49, 132.04, 133.94, 160.98.Catalytic performance of Pd-Cu bimetallic nanoparticle 4 for Heck ReactionIn an R.B flask under nitrogen atmosphere, a mixture of aryl iodide (1 mmol) with styrene or methylacrylate (1.2 mmol), Pd-Cu bimetallic nanoparticle 4 (1.5 mol %) and triethylamine (1.2 mL) was stirred in DMF (5 mL). The solution was heated for 24 hours at 90°C. The progress of the reaction was detected with the TLC chromatography (n-hexane/ethyl acetate 1:1). The reaction mixture was evaporated to dryness under reduced pressure after the desired transformation of the reaction was obtained and the residue was extracted with chloroform. The chloroform extract was washed with distilled water, dried over anhydrous Na2SO4, filtered, concentrated under reduced pressure and purified by silica gel column chromatography using ethyl acetate and hexane (3:1) to provide the desired products.Synthesis of (E)-Methyl 3-o-tolylacrylate 16White solid product, Yield: 96%, IR (KBr): δ max 2950.32, 1722.32, 1639.35, 1267.27, 1220.20, 1172.21, 980.30, 764.27. 1H NMR (400 MHz, CDCl3), δ 2.48 (s, 3H); 3.84 (s, 3H); 6.46 (d, 1H, J=16.0 Hz); 7.35-7.68 (m, 3H); 7.83-7.98 (m, 1H); 8.33 (d, J=8.0 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 19.66, 51.56, 118.46, 126.17, 126.61, 130.11, 130.75, 133.56, 137.66, 142.46, 167.36.Synthesis of trans stilbene 17, [39]White solid product, Yield: 94%, Colourless Solid was found and melting point was 74-76°C, IR (KBr): δ max 3027, 1600.35, 1496, 1452.24, 1319.36, 1267.27, 962.35, 909.25, 733.74. 1H NMR (400 MHz, CDCl3), δ 7.02 (s, 2 H), 7.35 (dd, 1 H, J=1.2 Hz, 7.2 Hz), 7.43 (dd, 4 H, J=9.2 Hz, 7.2 Hz), 7.63 (dd, 4H, J=1.2 Hz, 8.8 Hz). 13C NMR (100 MHz, CDCl3): δ 126.56, 127.61, 128.11, 128.85, 137.46.Synthesis of (E)-methyl 3-(4-methoxyphenyl)acrylate 18White solid product, yield: 97%, m. p. 135-137 oC, IR (KBr): δ max 2960.74, 1601.35, 1511.24, 1319.35, 1251.27, 1179.75, 1031.15, 966.36, 812.25. 1H NMR (400 MHz, CDCl3), δ 3.88 (s, 3H); 6.37 (d, 2H, J=8.0 Hz); 6.57 (d, 1H, J=16 Hz); 6.86 (d, 1 H, J=16 Hz); 6.39-6.29 (m, 1H); 7.58 (t, 2H, J=7.2 Hz); 7.94 (t, 4 H, J=9.2 Hz). 13C NMR (100 MHz, CDCl3): δ 55.31, 114.29, 126.32, 126.76, 127.26, 127.86, 128.33, 128.77, 130.22, 137.34, 159.40.
3. Results and Discussion
3.1. Synthesis and Characterization
- Dendrimer 3 was directly synthesized by a reaction of 2, 4, 6-triamino-1, 3, 5-Triazine 1 (1.59 mmol) with benzoyl chloride 2 (9.54 mmol) in anhydrous DMF at 80°C for 8 h in a nitrogen atmosphere (Scheme 1). The movement of the reaction was detected by thin layer chromatography (TLC) and after complete transformation of the reaction, the dendrimerized product was found by purification of the solid reaction mixture with recrystallization. The product was analyzed by IR, 1H NMR, 13C NMR (S1-S3 in the supporting information).
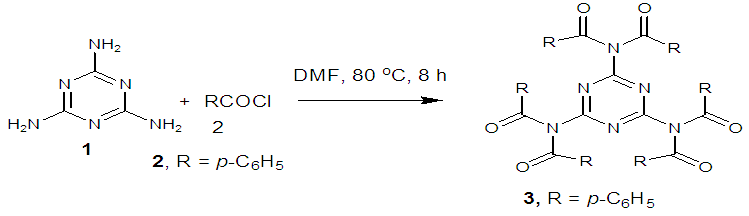 | Scheme 1. Synthesis of dendrimer 3 |
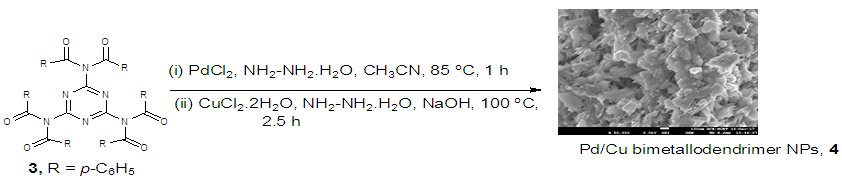 | Scheme 2. Synthesis of Pd-Cu bimetallic nanoparticle 4 |
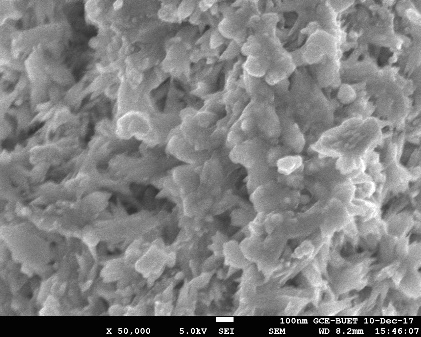 | Figure 1. SEM images of the nanoparticle 4 |
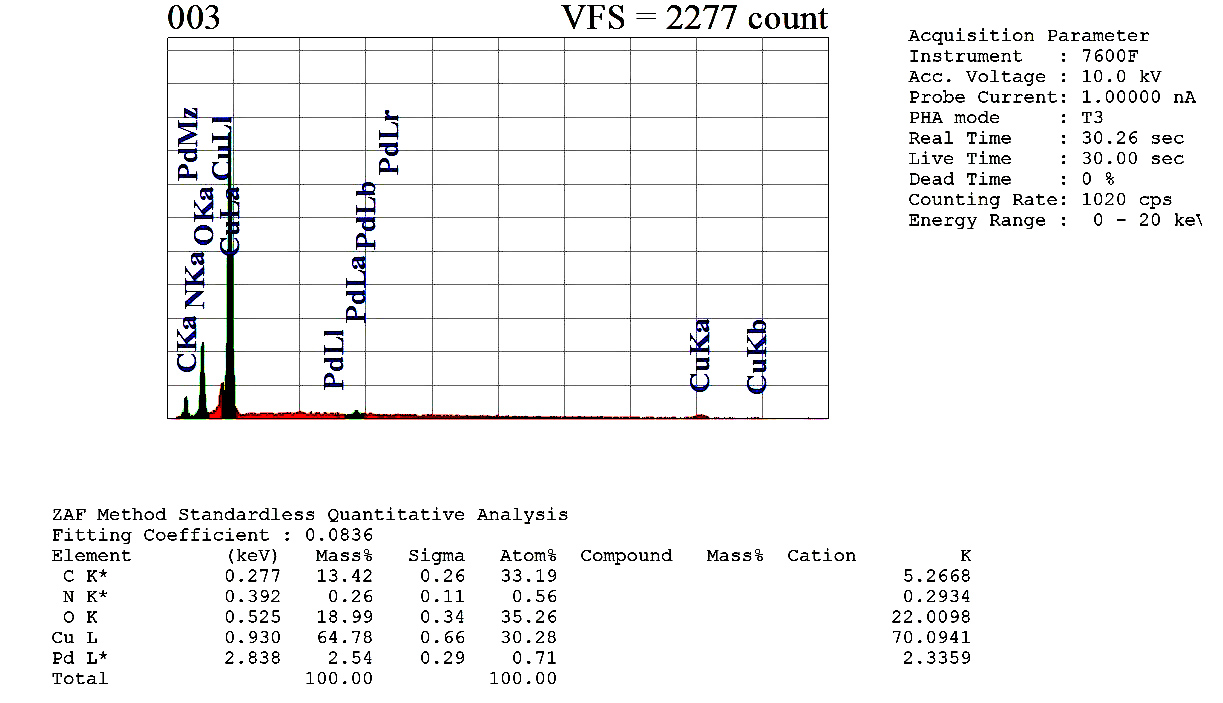 | Figure 2. EDX analysis of the nanoparticle 4 |
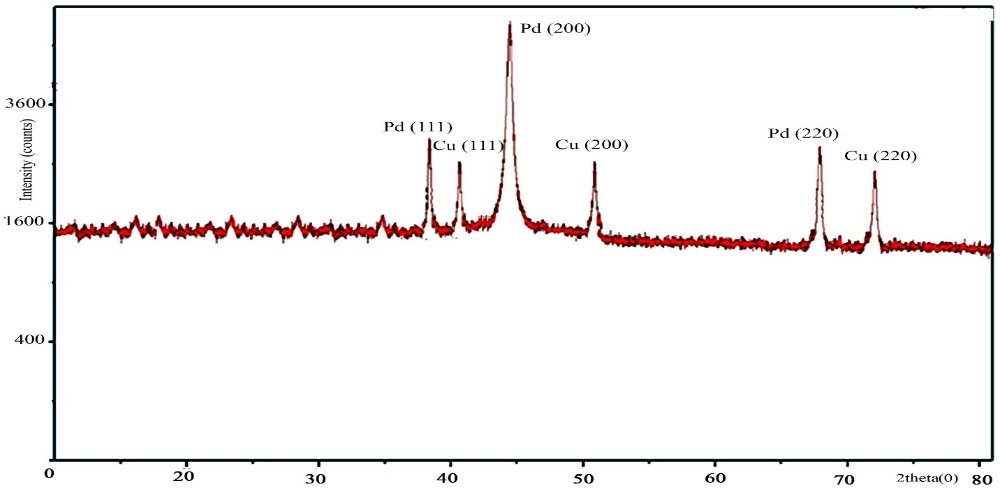 | Figure 3. XRD of Pd-Cu bimetallic nanoparticle 4 |
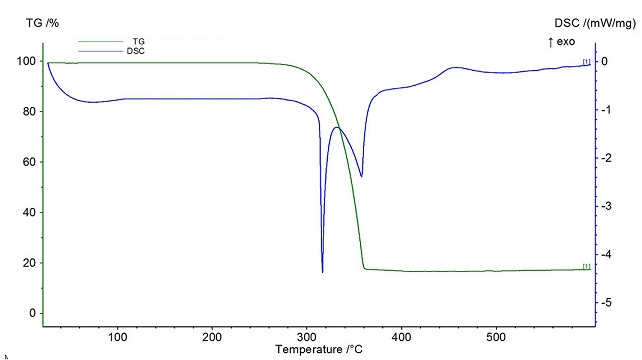 | Figure 4. DSC & TGA of Pd-Cu bimetallic nanoparticle 4 |
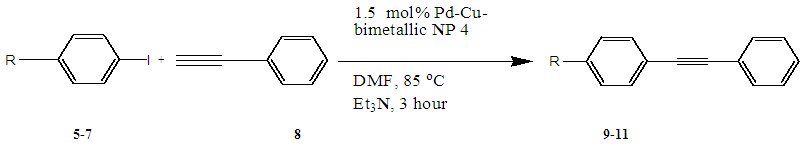
3.2. Catalytic Performance of Pd/Cu Bimetallic Nanoparticle 4 in Sonogashira and Heck Reaction
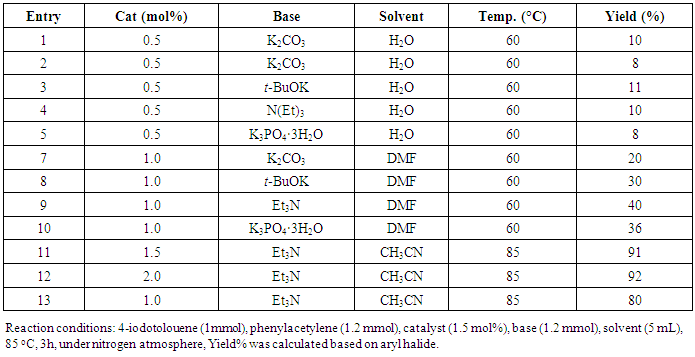
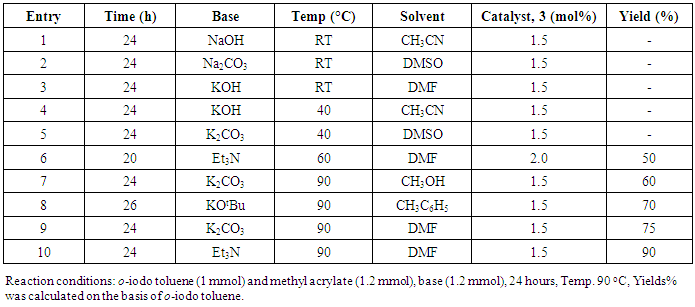
- In the case of Pd-Cu bimetallic NPS, the synergistic effect between palladium and copper showed astonishing efficiency for the various carbon-carbon cross-coupling reactions [42]. In the synergistic mechanism, the energy gap between HOMO and LUMO comes closer by two metal catalyst stimulating both nucleophile and electrophile simultaneously [43].The catalytic activity of synthesized Pd-Cu bimetallic nanoparticle 4 catalyst was studied in Sonogashira alkynylation reaction of aryl halides with terminal alkynes.A typical reaction between the reaction of 4-iodotoluene and phenylacetylene and the impact of various factors along with the solvent, reaction temperature and catalyst loading was studied due to the determination of optimized reaction conditions. This reaction gave very low yields with water as the solvent and distinctive bases together with Na2CO3, K2CO3, t-BuOK, Et3N, and K3PO4 (Table 1, entries 1–5) in the presence of 0.5 mol % Pd-Cu bimetallic NP 4. The yields were advanced with the help of changing the solvent to DMF and using different bases (Table 1, entries 6–10) in the presence of a 1 mol % catalyst. Curiously, by increasing the temperature of the reaction to 85°C and catalyst to 1.5 mol %, the best results with a yield of 91% were obtained by using Et3N as a base, CH3CN as a solvent (Table 1, entry 11). However, with a view to confirming approximately catalyst loading, this reaction was performed in the presence of 2.0 mol % and 1.0 mol % of catalyst 4 and results showed the formation of preferred products in 92% and 80% yield respectively (Table 1, entries 12 and 13). For this reason, we selected 1.5 mol % of catalyst, Et3N as base and CH3CN as solvent at 85 °C at 3h for maximum efficiency and optimized reaction conditions (Table 1, Entry 11).
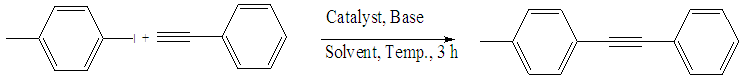 | Scheme 3. Optimization of Pd-Cu bimetallic nanoparticle 4 catalyzed between 4-iodotoluene and phenylacetylene |
|
 | Scheme 4 |
|
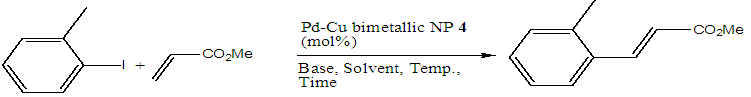 | Scheme 5. Optimization of the Pd-Cu bimetallic NP 4 catalyzed Heck reaction between o-iodo toluene and methyl acrylate |
|
|
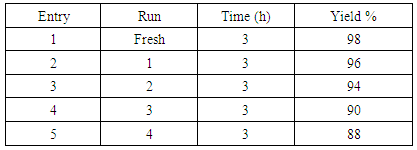
3.3. Reusability and Stability of Recovered Pd-Cu Bimetallic NPs 4
- The recyclability of Pd-Cu bimetallic NPs 4 to the coupling reaction between 4-iodotoluene and phenylacetylene was also studied (Table 5). After the reaction, black Pd-Cu bimetallic NP 4 was simply recovered from the reaction mixture after each catalytic run by centrifugation, filtration, thoroughly washed with water followed by acetone, and then dried under vacuum.
|
 | Figure 5. SEM image of the recovered NP 4 |
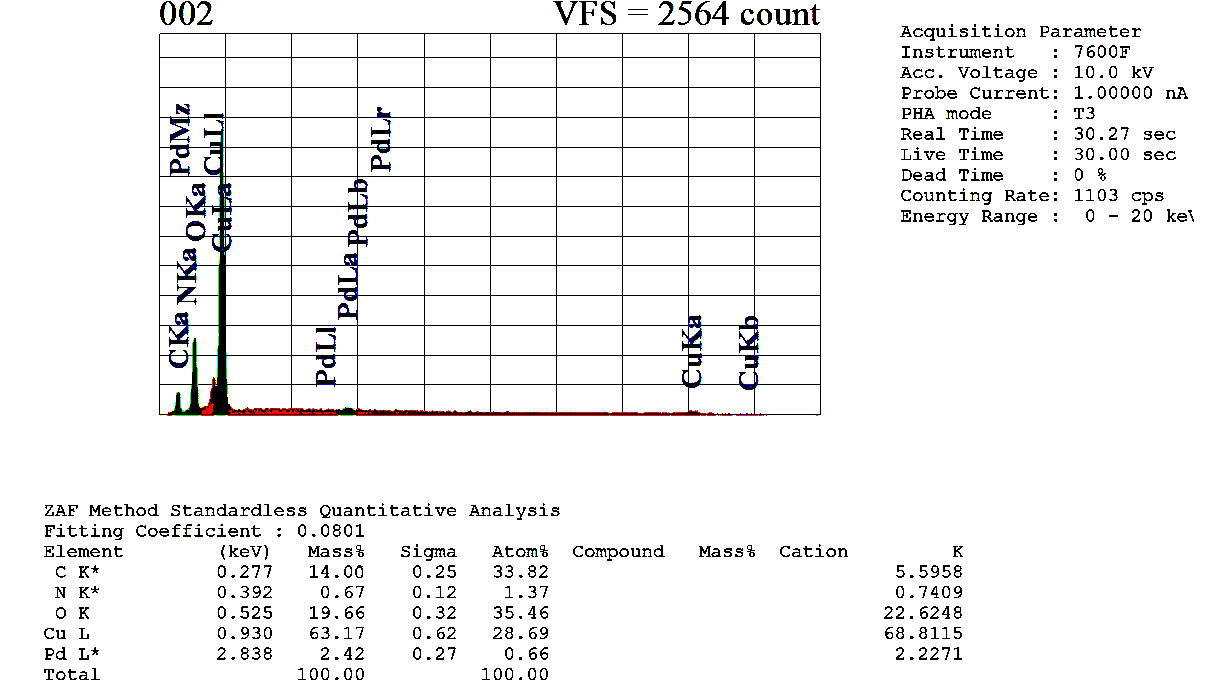 | Figure 6. EDX analysis of the recovered NP 4 |
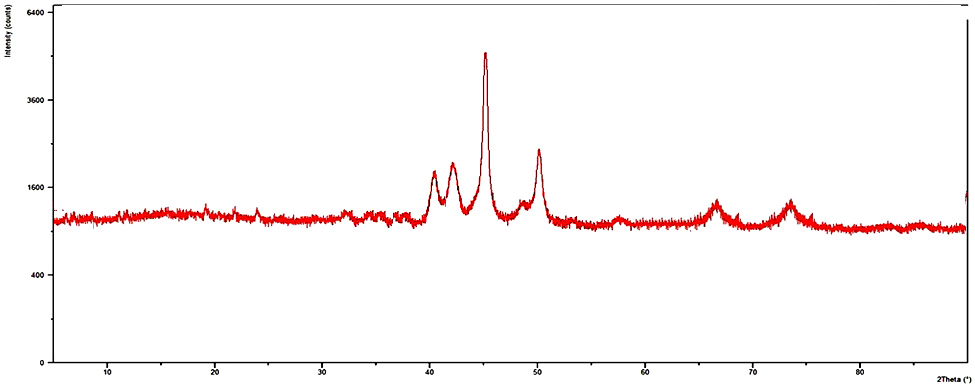 | Figure 7. XRD analysis of the recovered NP 4 |
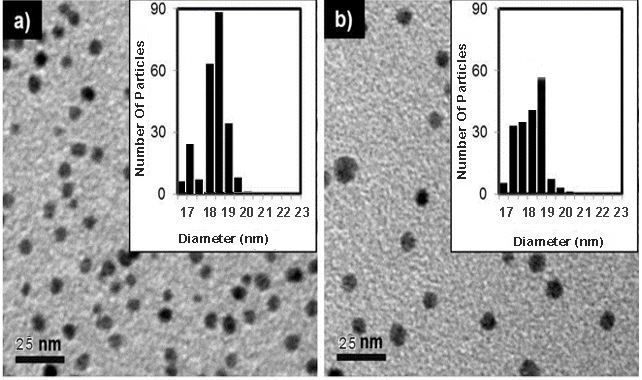 | Figure 8. TEM analysis of the pure Pd-Cu bimetallic NPs 4 (Fig. 8a) and recovered NP 4 (Fig. 8b) |
4. Conclusions
- In summary, we have synthesized triazine-based dendrimer assisted heterogeneous Palladium-Copper bimetallic nanoparticles by sequential loading method. Because of its low rate, however, most effective palladium with bimetallic nanocatalyst and fantastically valuable, recoverable, reusable, a small amount of palladium salt was used as copper salt. All analytical consequences (SEM, EDX, XRD, TG&DSC, TEM and leaching study) verify their heterogeneous catalytic activity in the carbon-carbon cross-coupling reaction such as Heck, Sonogashira and the synthetic route used to be also discovered to be phosphine ligand-free. Further applications of this heterogeneous catalytic activity in a number of C–C coupling reactions such as Stile, Suzuki coupling are being investigated.
ACKNOWLEDGMENTS
- We thank the Ministry of Science and Technology, Dhaka, Bangladesh (National Science & Technology Fellowship Program 2018-2019, Ph.D. Fellowship, No- 39.00.0000.012. 002.03.18. 25, Code No-1260101-120005100-3821117) and the University of Engineering and Technology of Bangladesh (BUET), Dhaka, Bangladesh for providing financial support for our research work.
Supporting Information
- IR spectra of 2, 4, 6-Tris (di-benzamido)-1, 3, 5-triazine 3
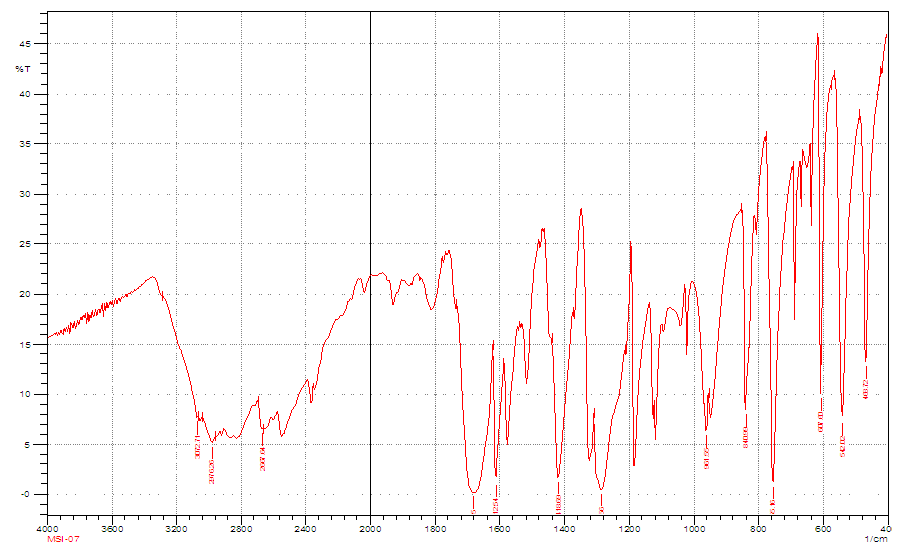 | Figure S1 |
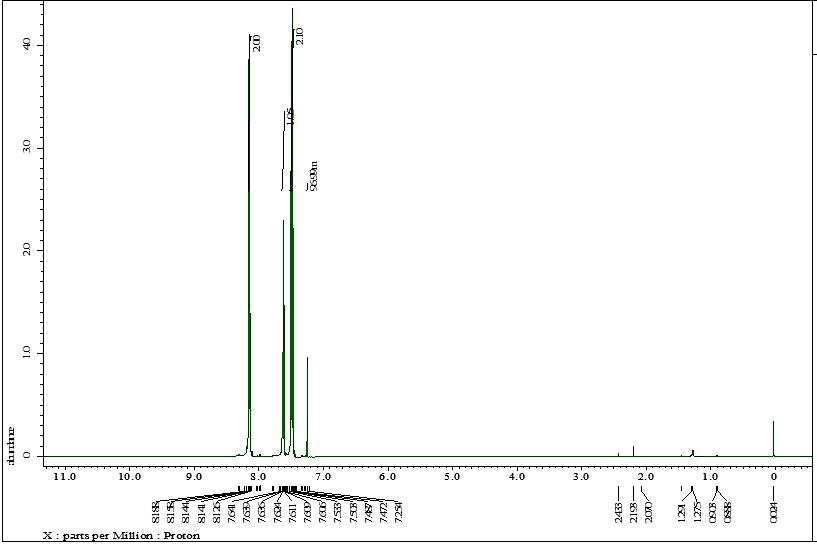 | Figure S2 |
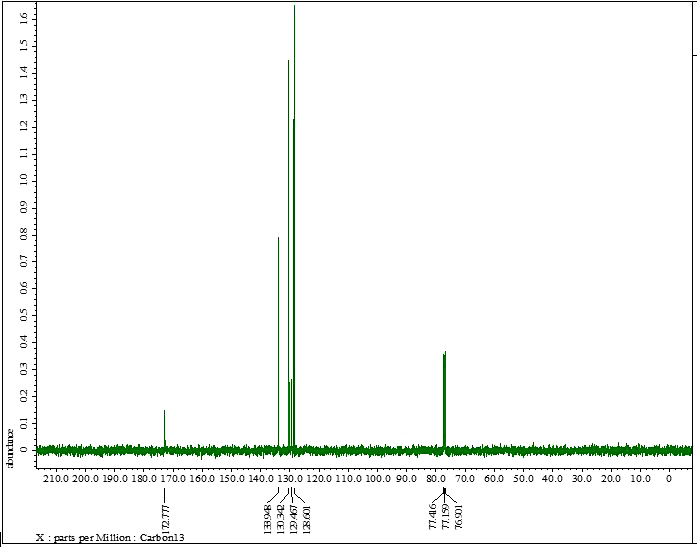 | Figure S3 |
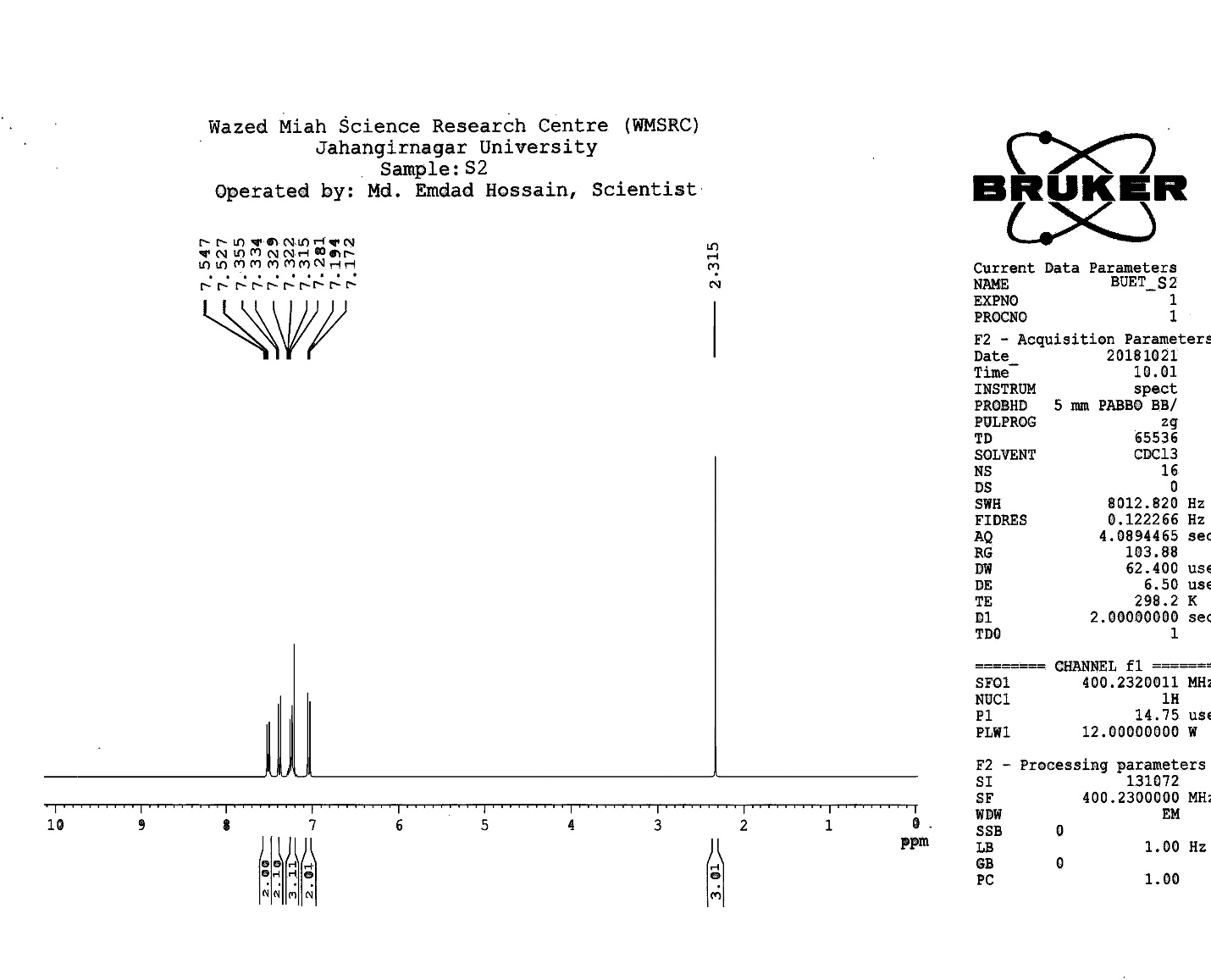 | Figure S4 |
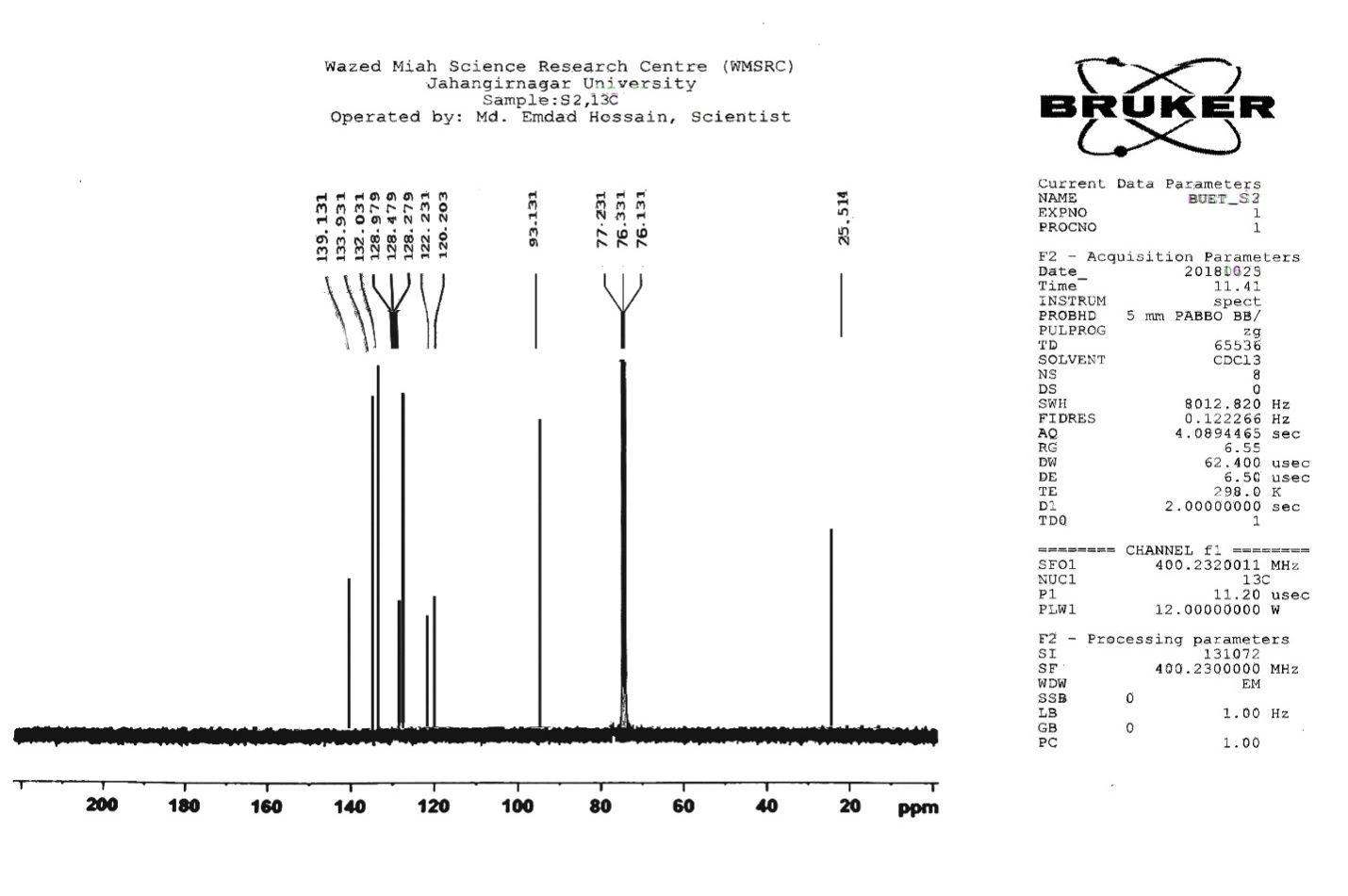 | Figure S5 |
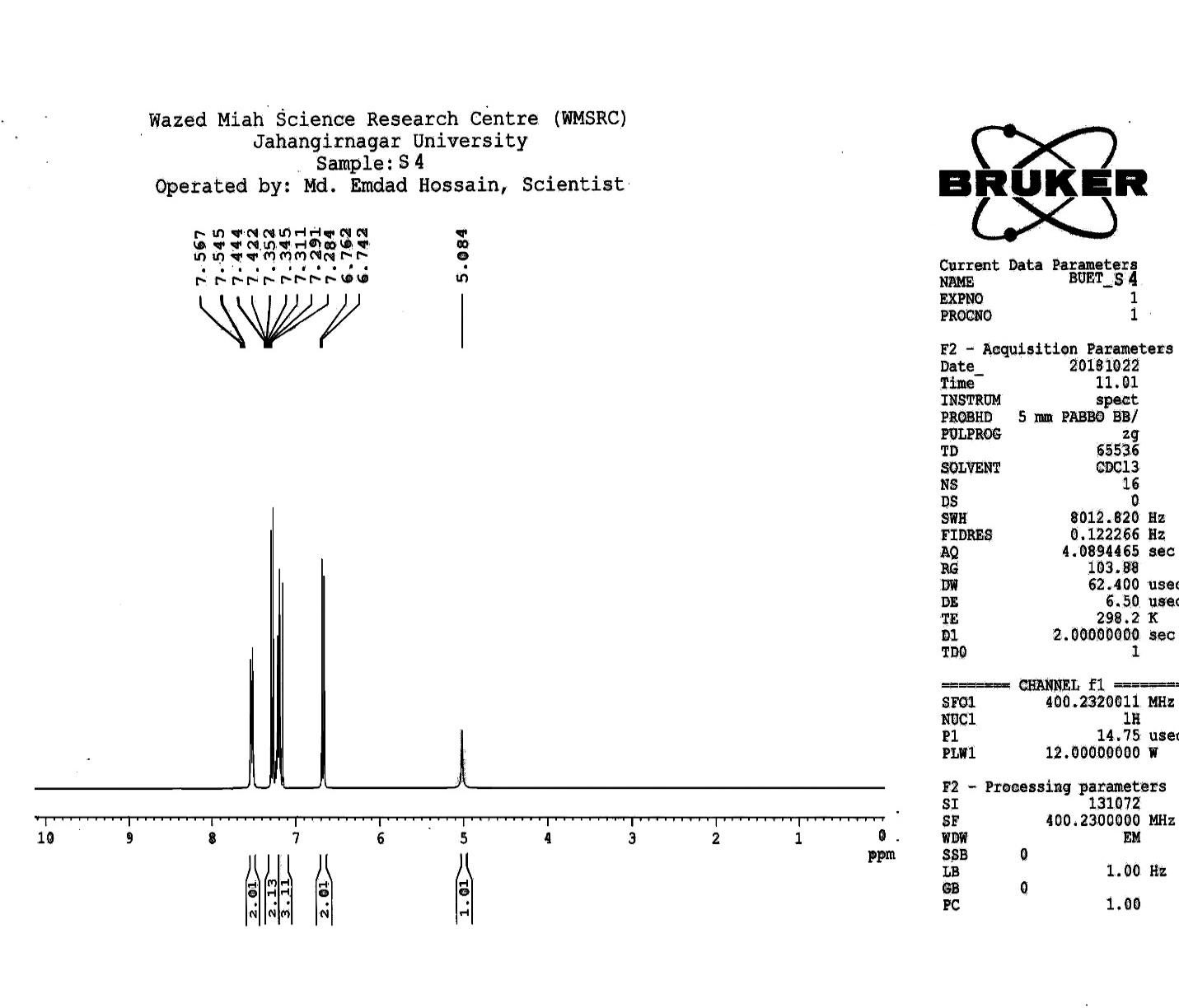 | Figure S6 |
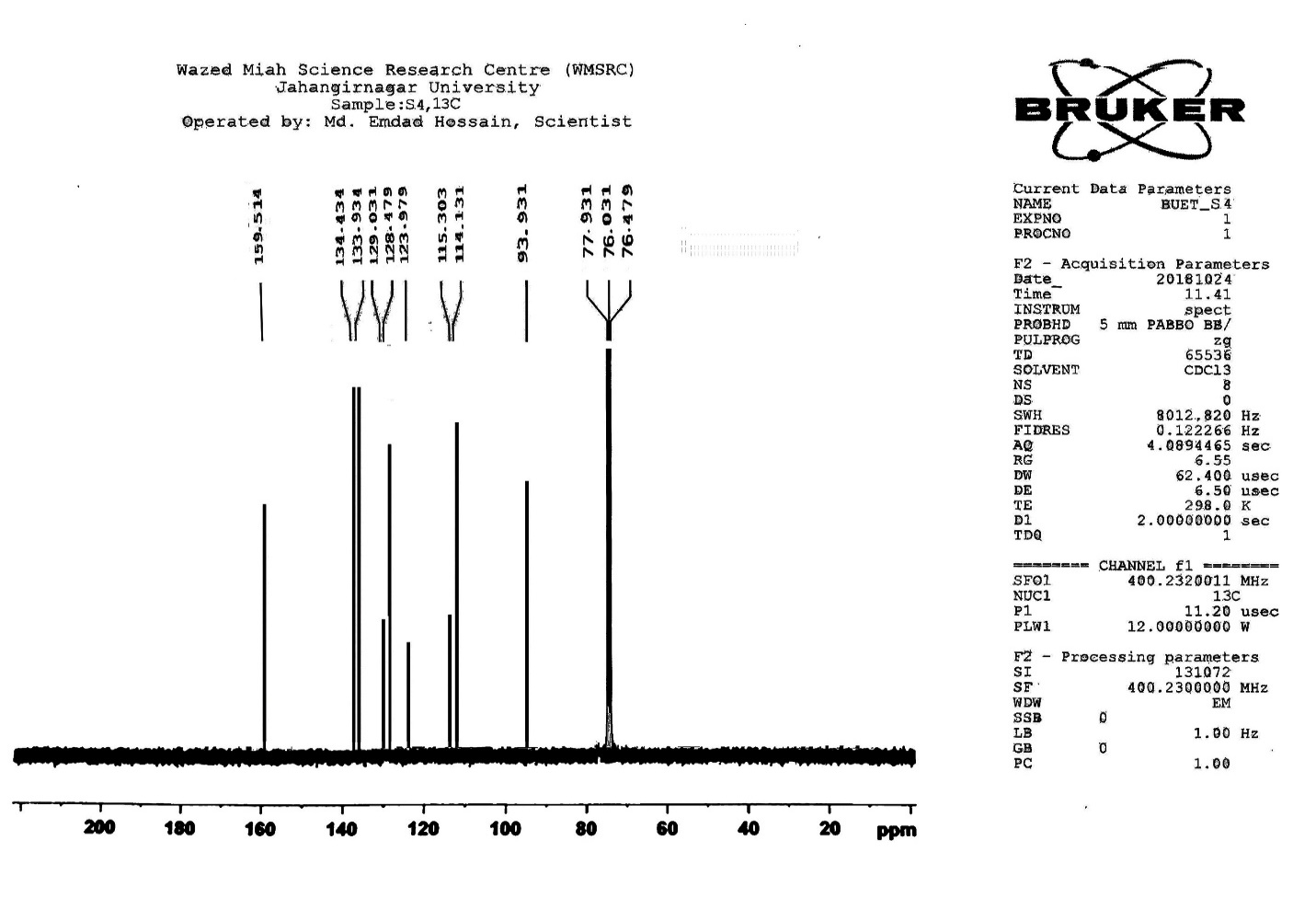 | Figure S7 |
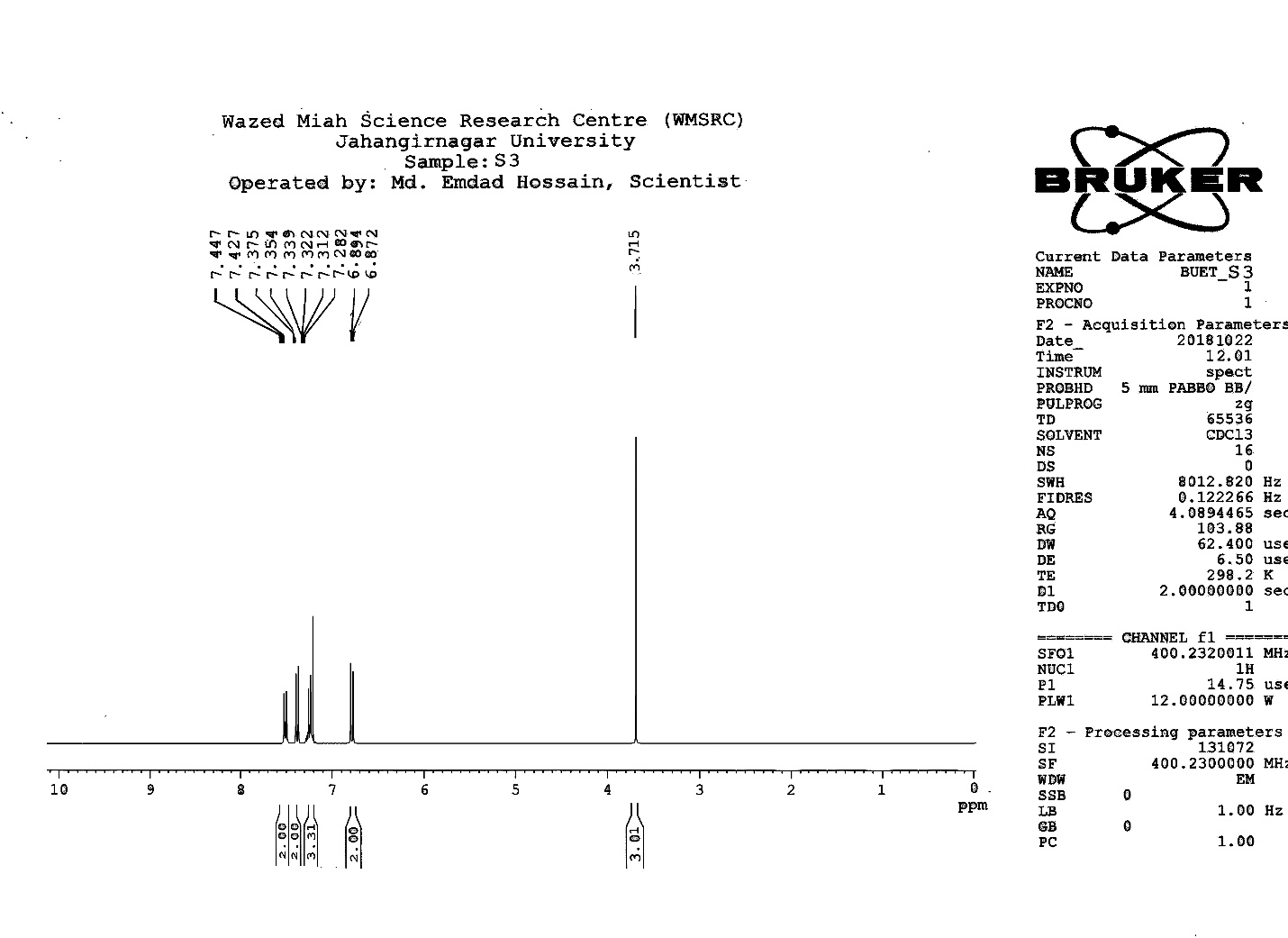 | Figure S8 |
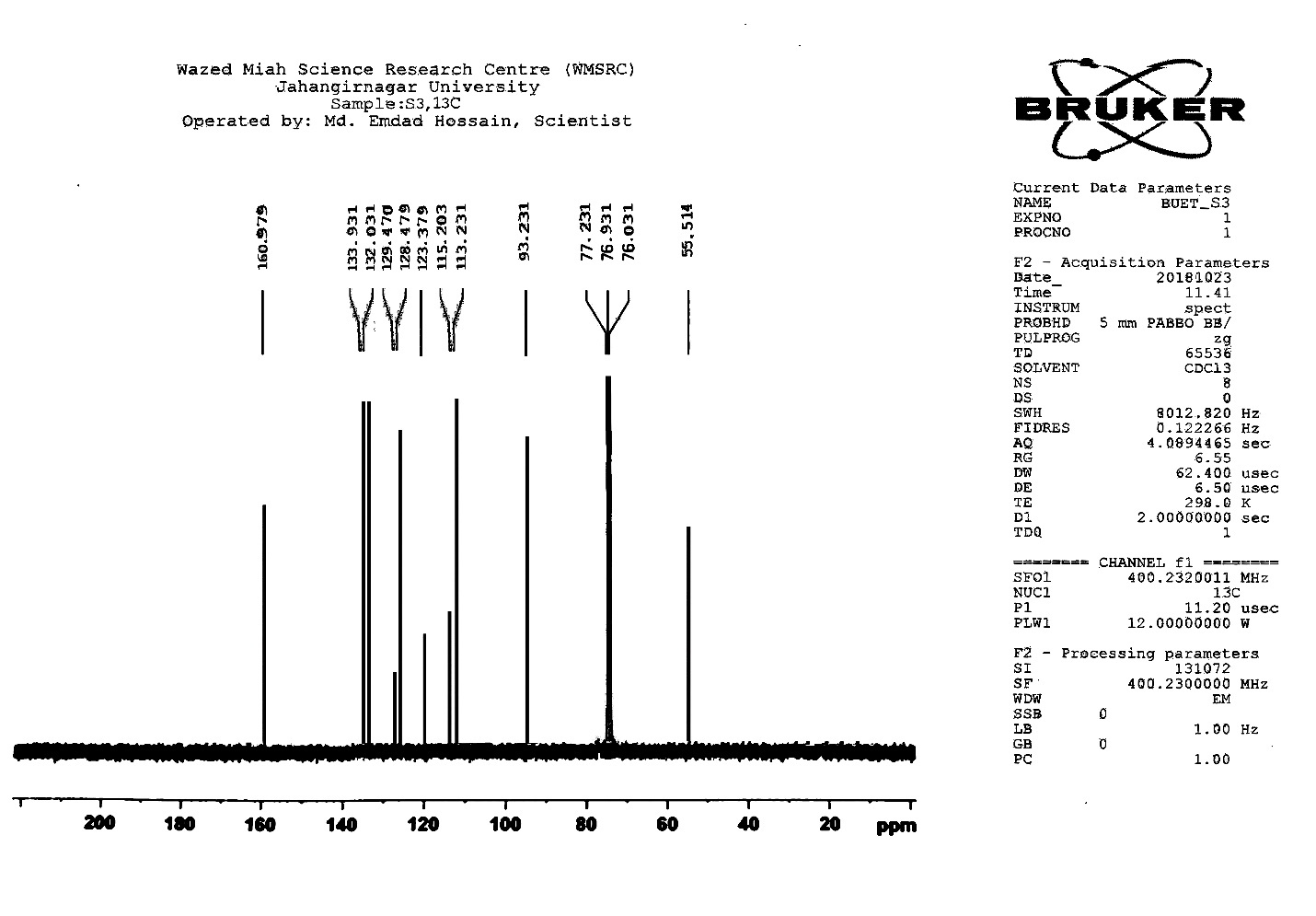 | Figure S9 |
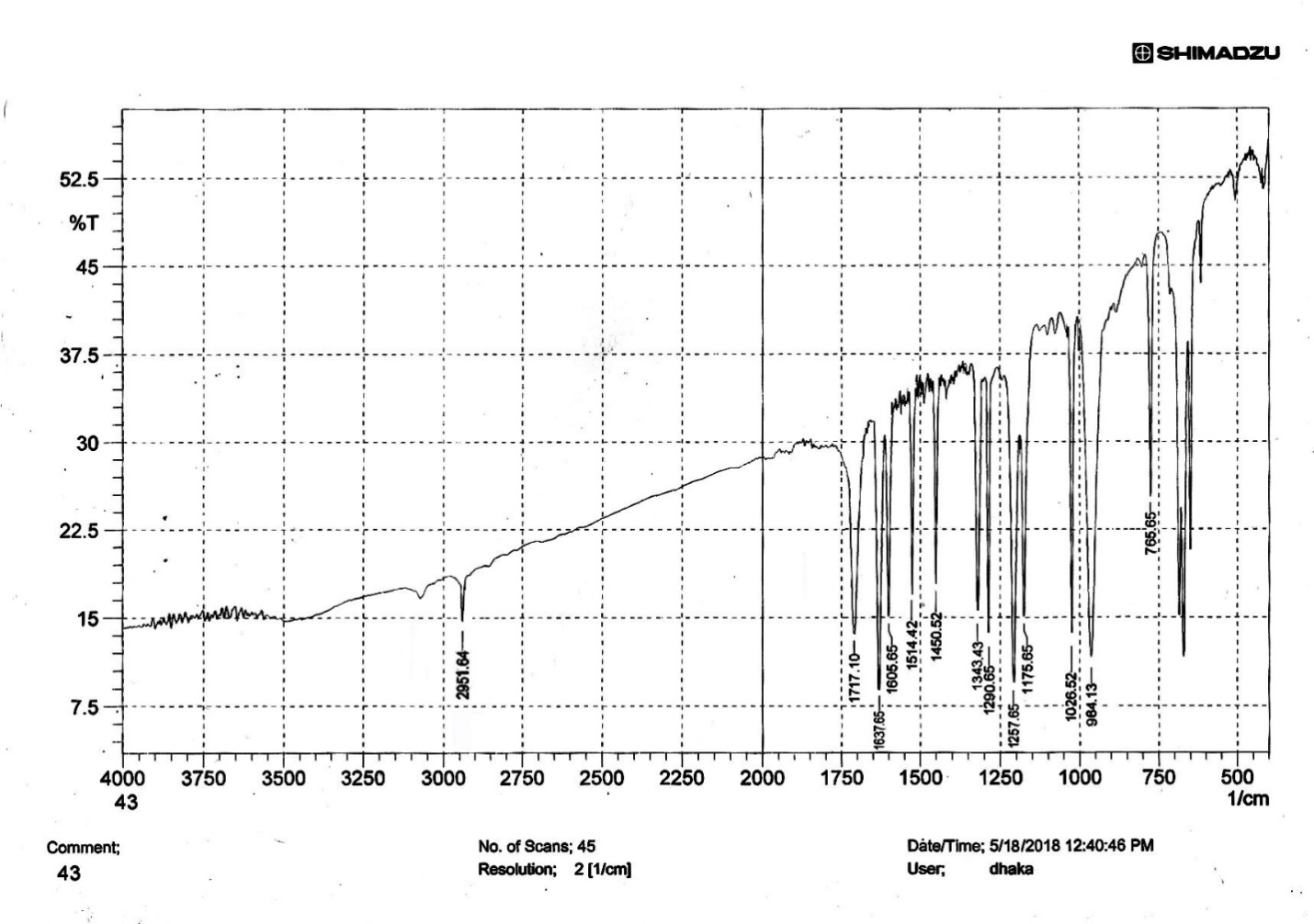 | Figure S10 |
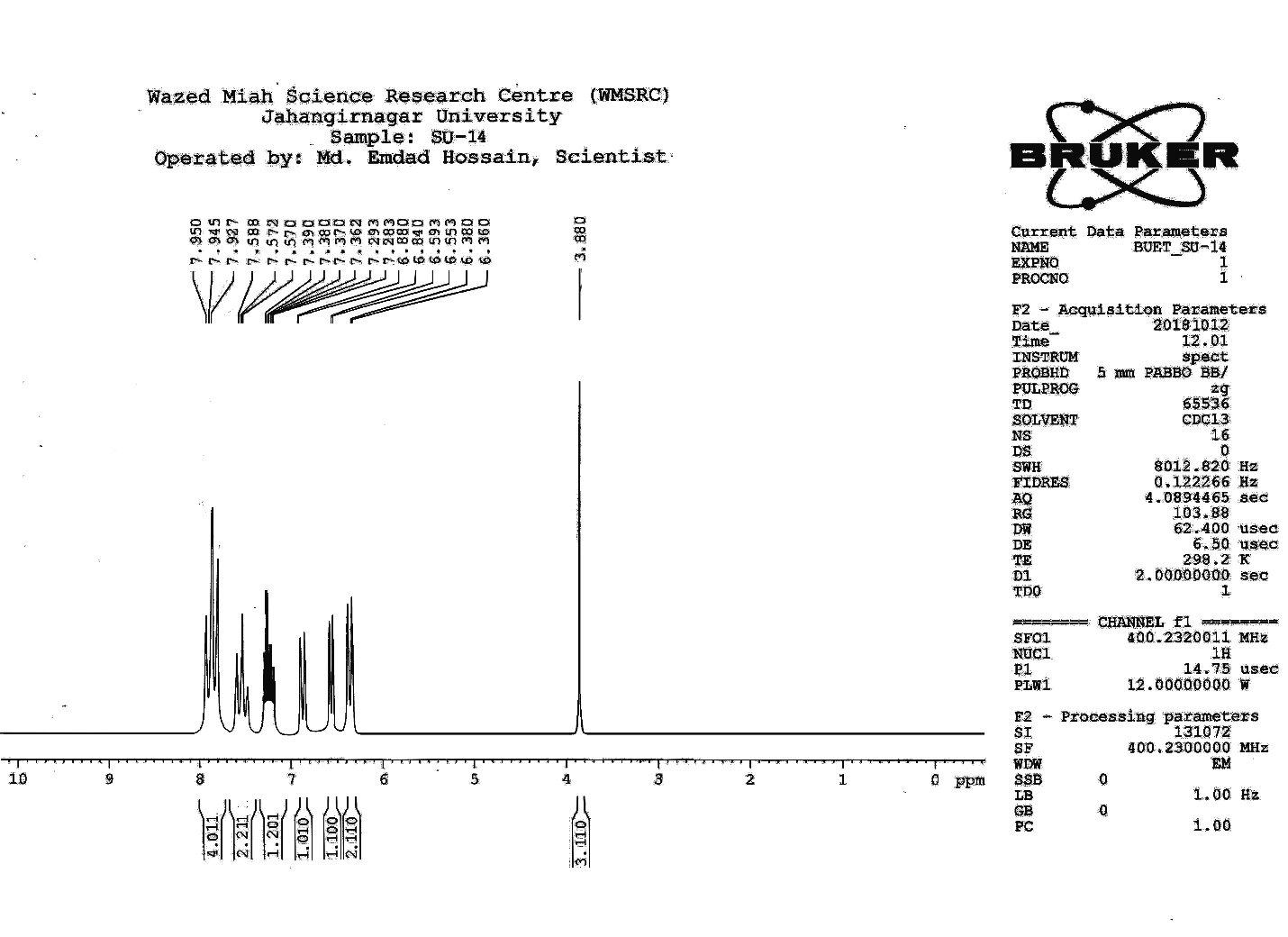 | Figure S11 |
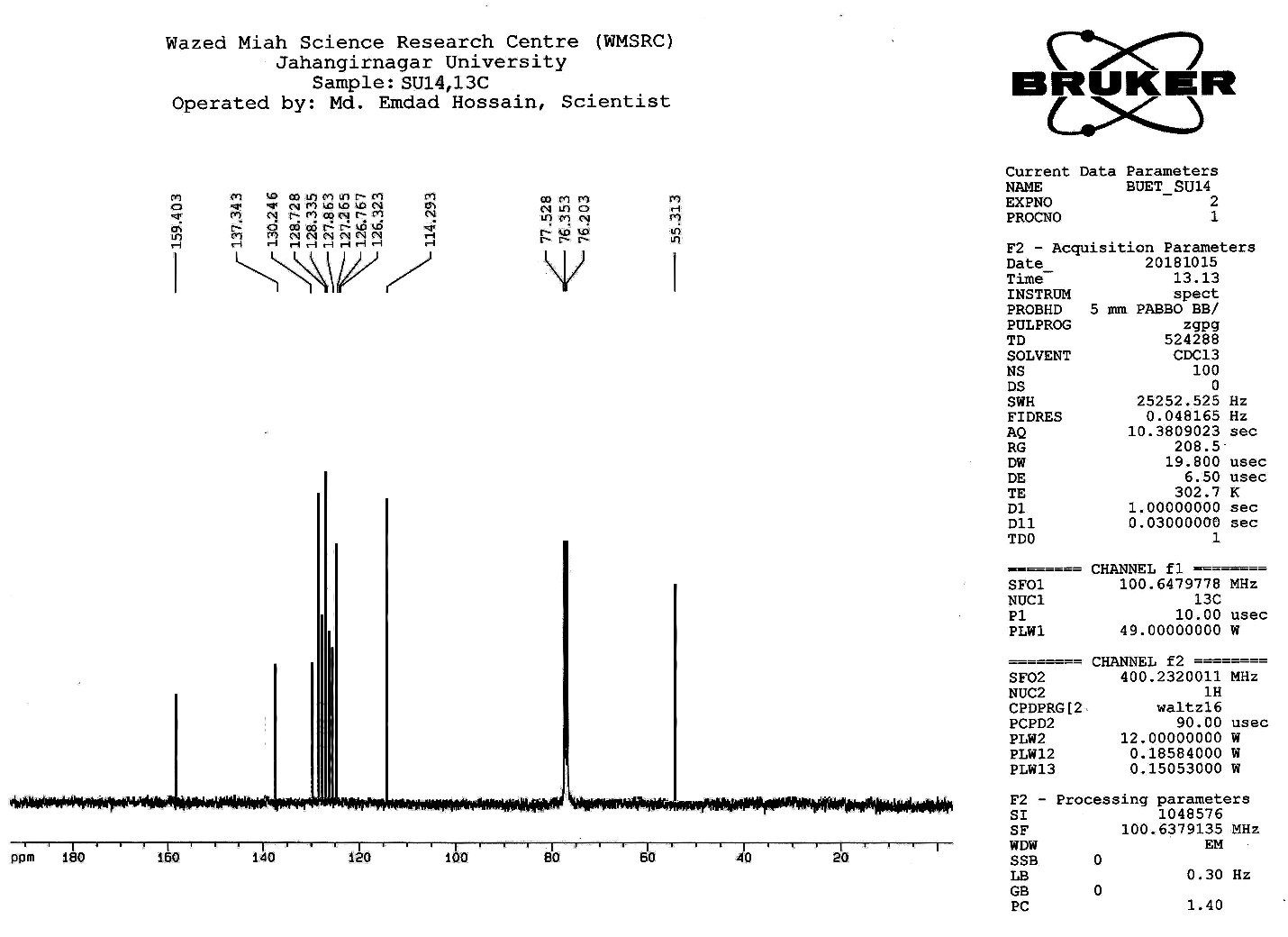 | Figure S12 |
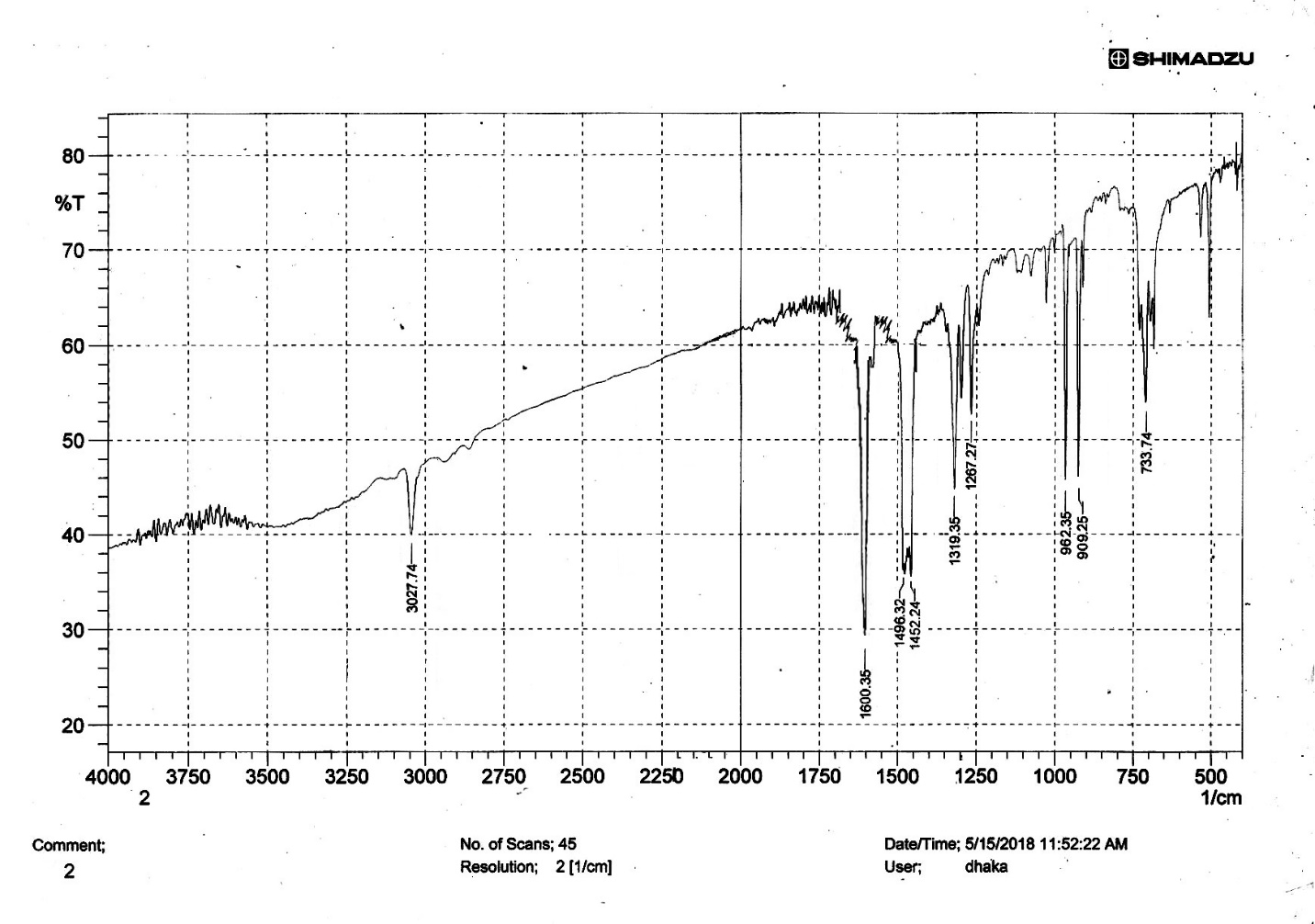 | Figure S13 |
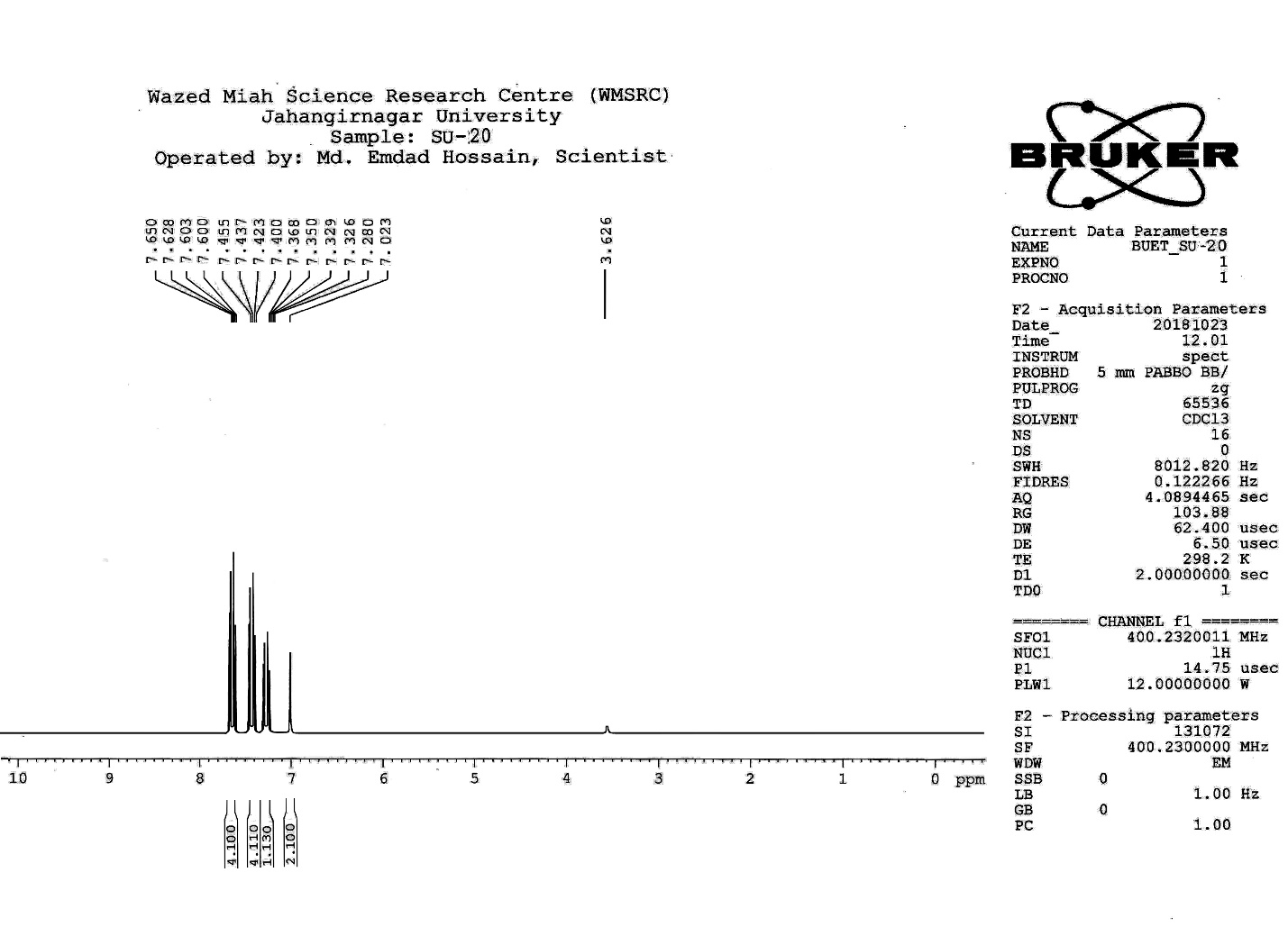 | Figure S14 |
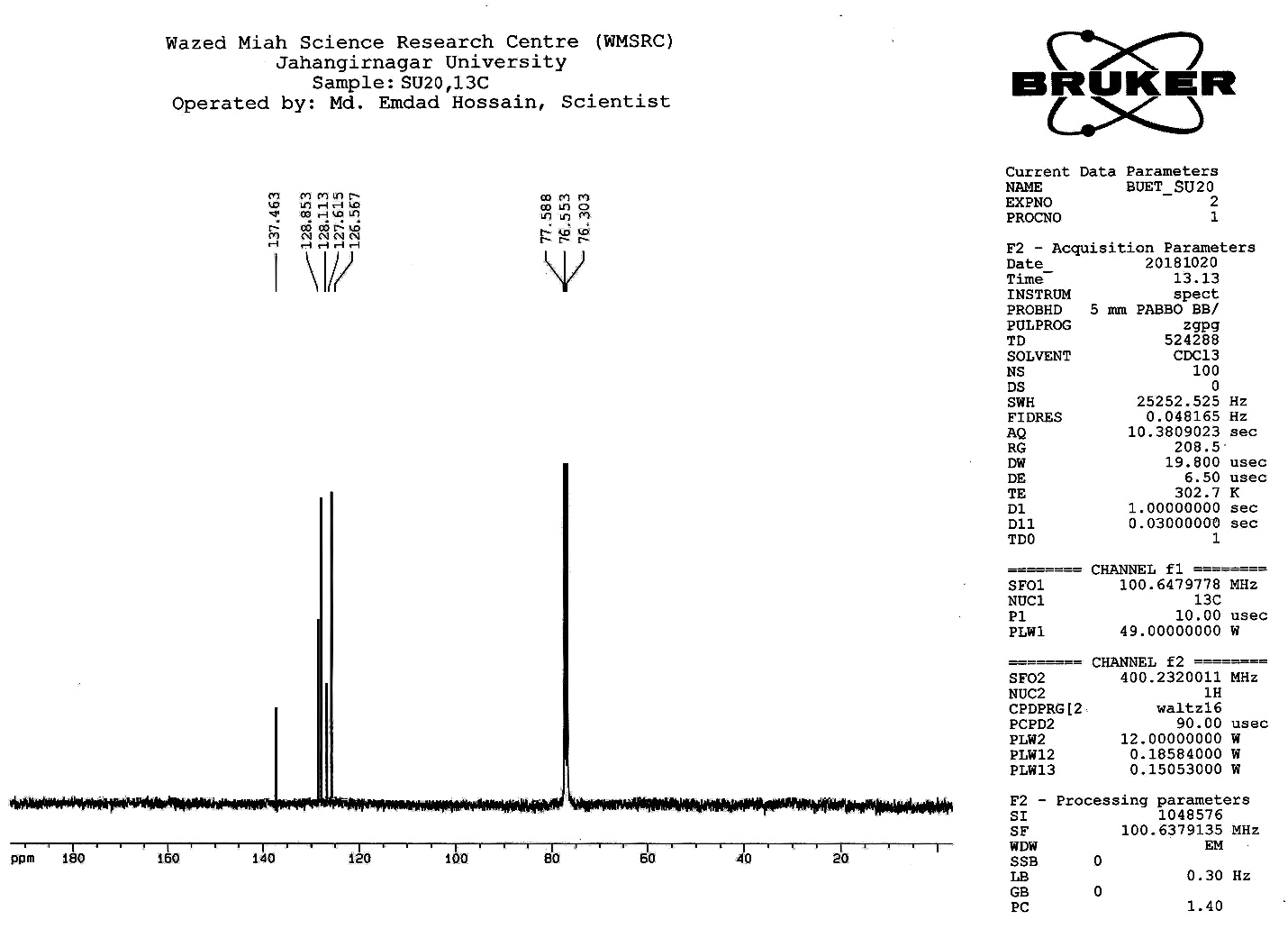 | Figure S15 |
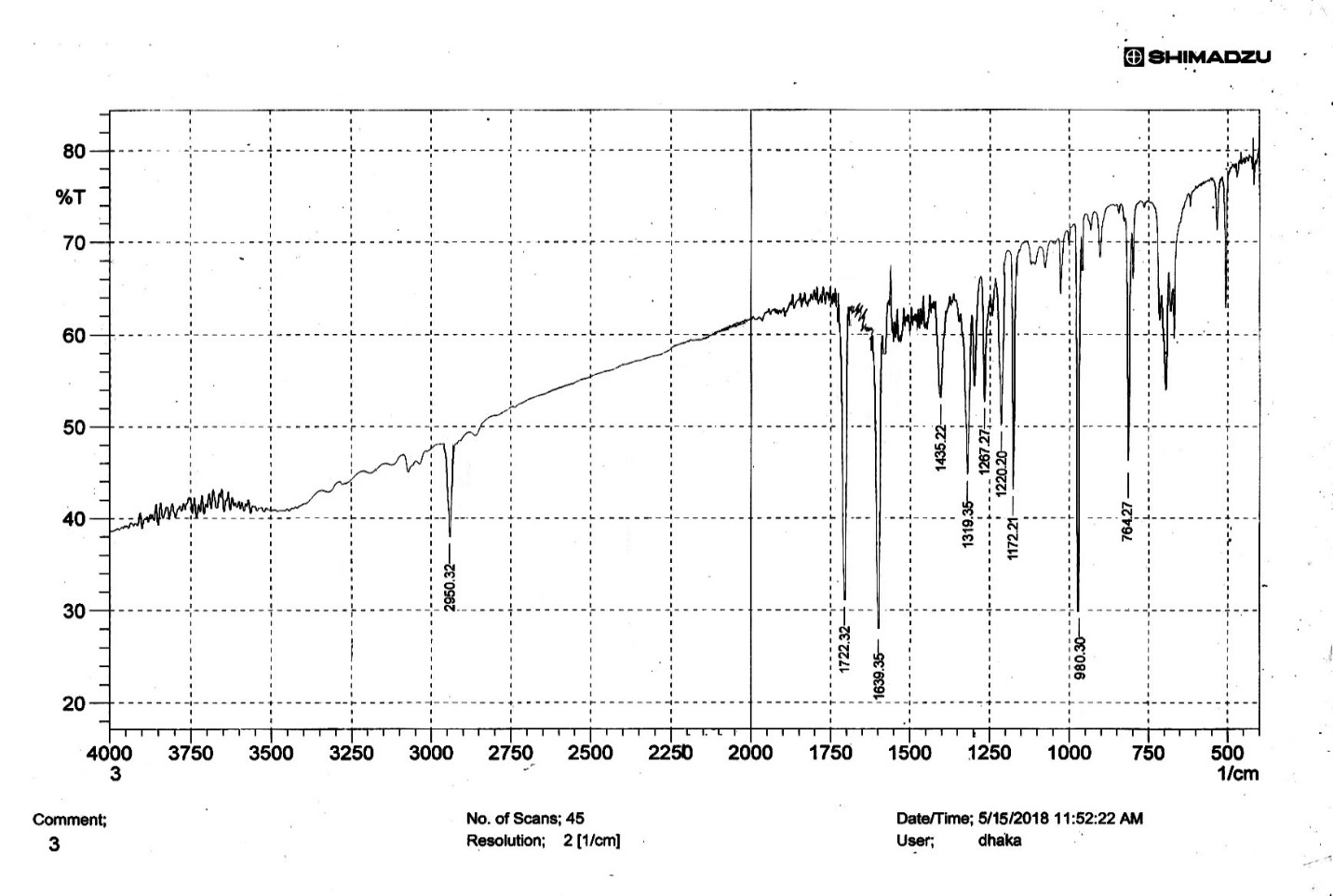 | Figure S16 |
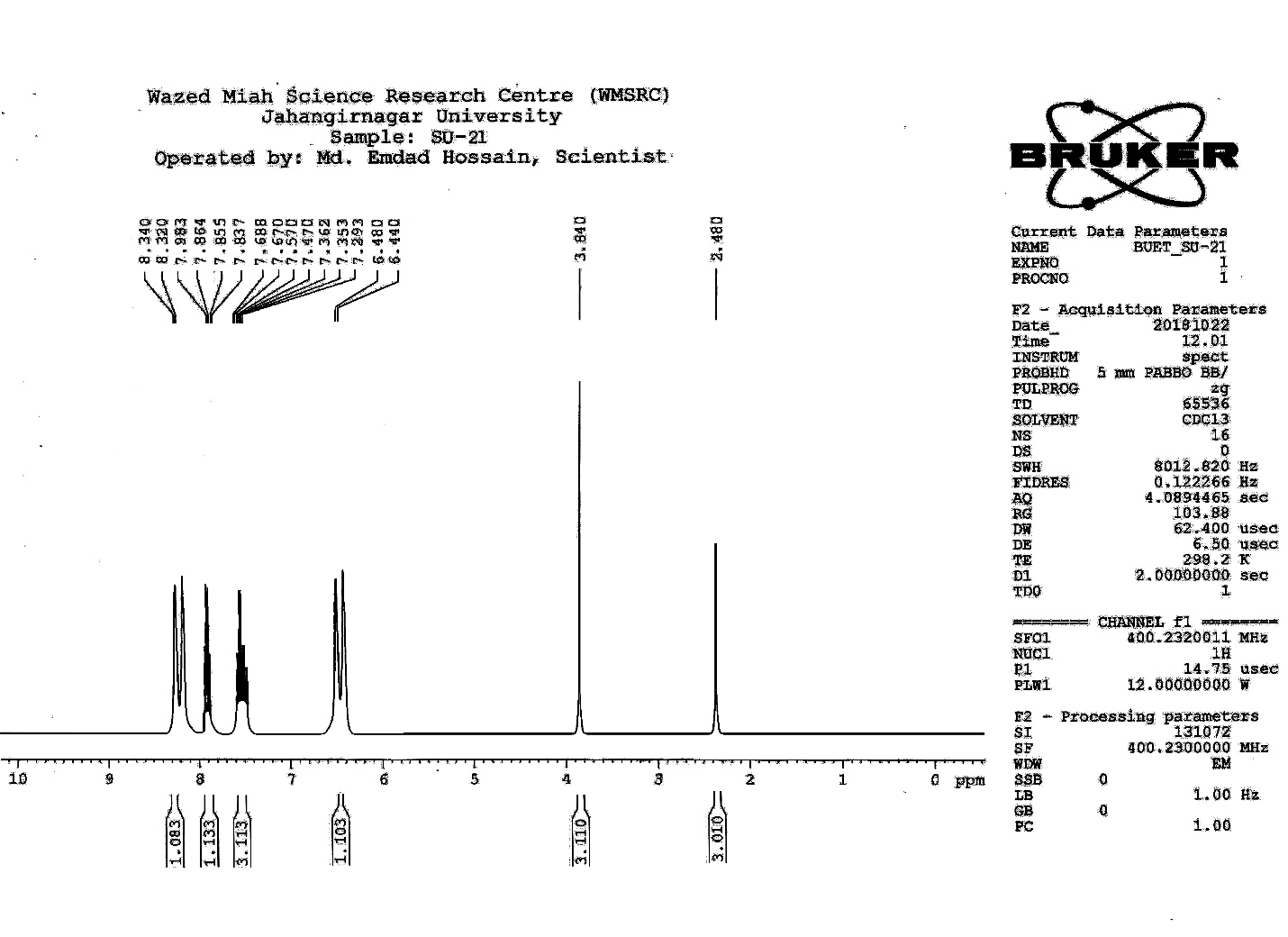 | Figure S17 |
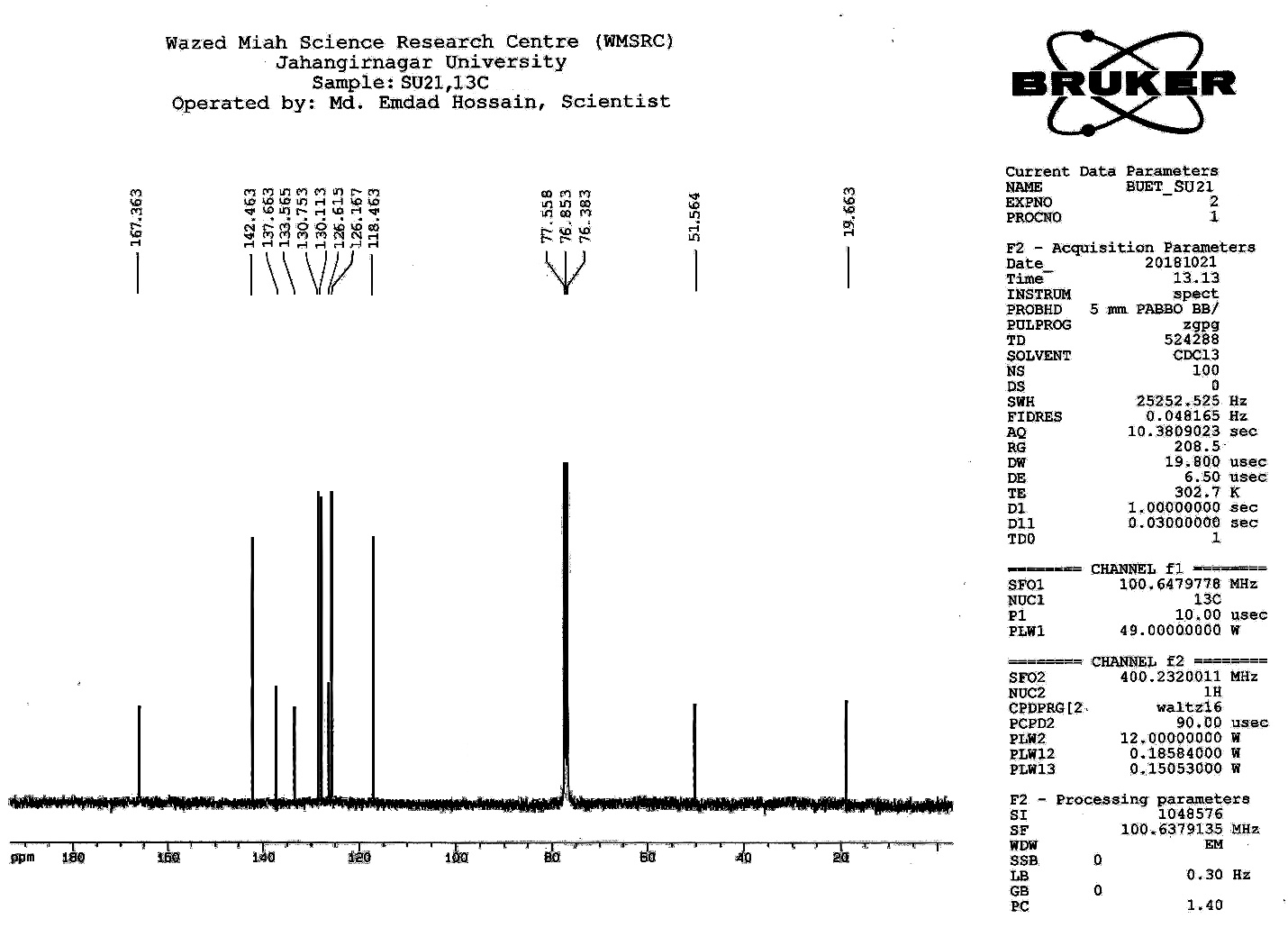 | Figure S18 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML