-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2018; 8(2): 25-30
doi:10.5923/j.nn.20180802.01

Serum Albumin Protein-Mediated Ultra-Fast Laser Synthesis of Calcium Phosphate Nanoceramic in Aqueous Solution
Marina Rodio1, 2, Romuald Intartaglia3
1Hamburg Centre for Ultrafast Imaging, Luruper Chaussee 149, Hamburg, Germany
2Physical Chemistry, Hamburg University, Martin-Luther-King Platz 6, Hamburg, Germany
3Nanophysics, Istituto Italiano di Tecnologia, Via Morego 30, Genoa, Italy
Correspondence to: Romuald Intartaglia, Nanophysics, Istituto Italiano di Tecnologia, Via Morego 30, Genoa, Italy.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

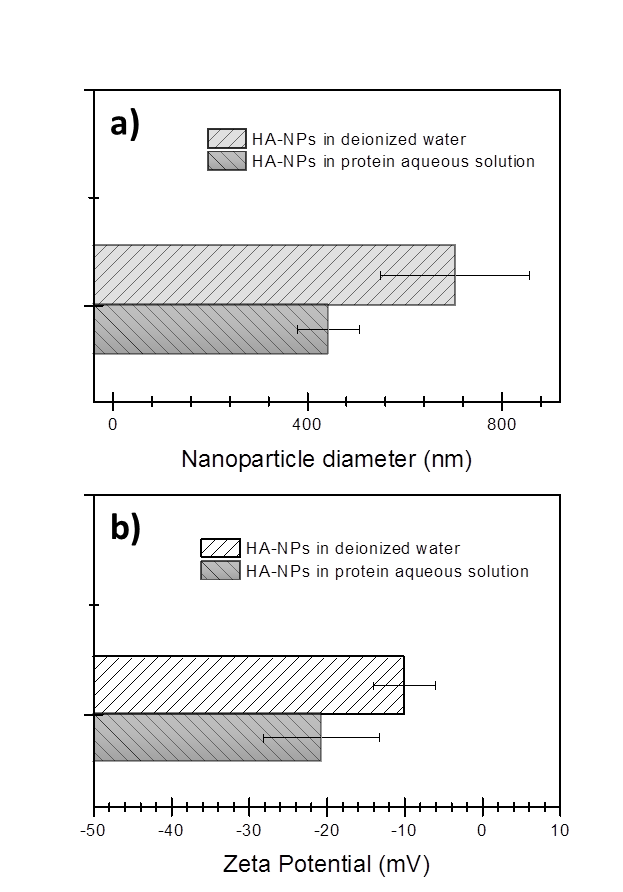
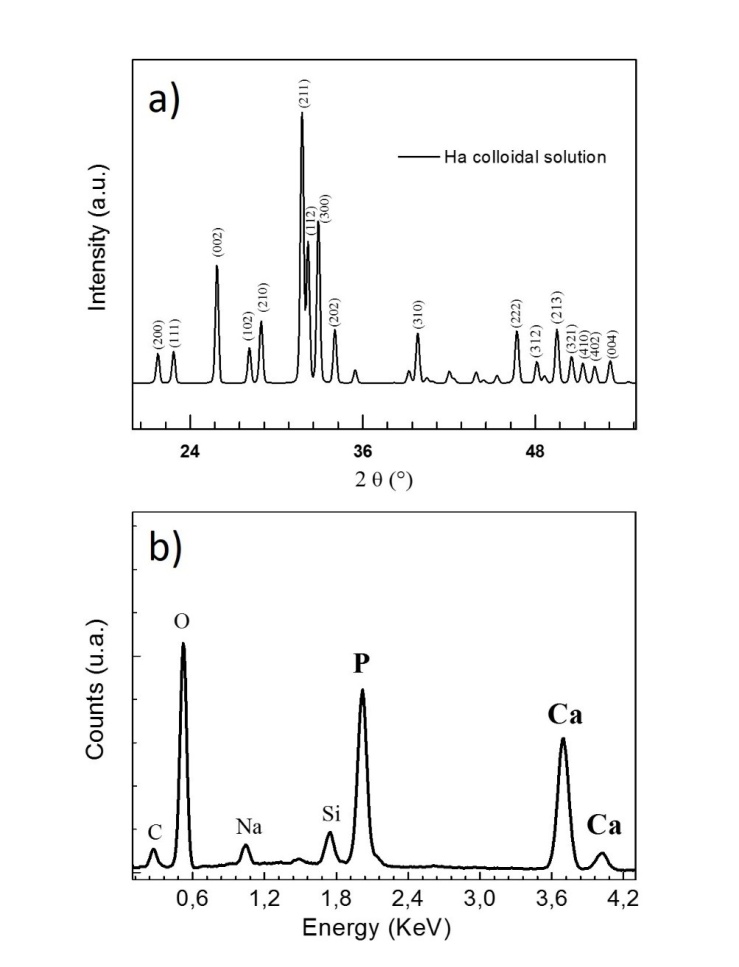
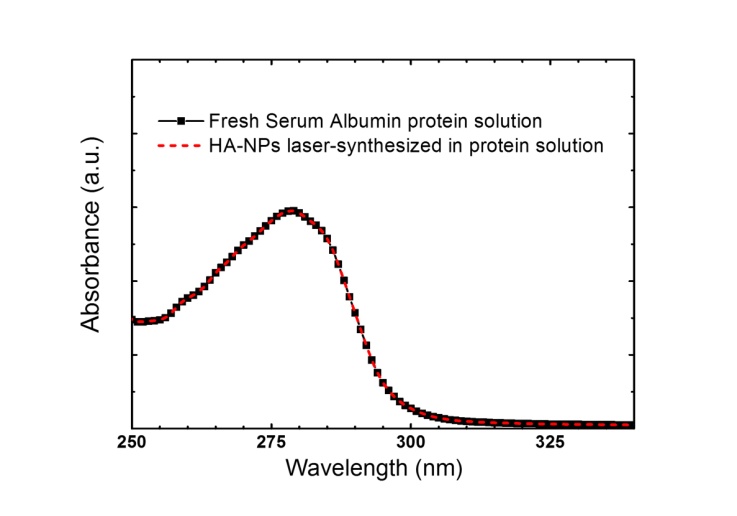
Ceramic nanoparticles are promising biomaterials for bone tissue regeneration, cell proliferation and as plasmid DNA delivery vector, because they bear outstanding properties of chemical similarity to the human mineral constituent, bioactivity, and are fairly easily bio-conjugated. Here we report on the synthesis of hydroxyapatite nanoparticles by laser ablation of hydroxyapatite target in deionized water and in aqueous solution of Serum Albumin protein. Laser ablation in deionized water results in the formation of large clusters composed by small nanoparticles, while in Serum Albumin protein solution a starting process of clusters disaggregation is observed. Stability and nanoparticle size-quenching effect is investigated by various methods such as electron microscopy zeta potential and dynamic light scattering. It is found that the presence of Serum Albumin protein in aqueous solution plays the role of size-regulating agent. The integrity of Serum Albumin protein after laser irradiation is assessed by means of UV-vis and FT- IR analysis. Further, the chemical structure and crystallinity of the hydroxyapatite colloidal solution is investigated by infrared spectroscopy, X-Ray diffraction measurement and energy-dispersive X-ray diffraction.
Keywords: Calcium phosphate, Hydroxyapatite, Serum Albumin Protein, Laser Synthesis in Liquid, Green synthesis
Cite this paper: Marina Rodio, Romuald Intartaglia, Serum Albumin Protein-Mediated Ultra-Fast Laser Synthesis of Calcium Phosphate Nanoceramic in Aqueous Solution, Nanoscience and Nanotechnology, Vol. 8 No. 2, 2018, pp. 25-30. doi: 10.5923/j.nn.20180802.01.
Article Outline
1. Introduction
- Hydroxyapatite nanoparticles (HA-NPs) are promising biomaterials due to their outstanding properties[1] of biocompatibility, biodegrability, bioactivity, and chemical and structural similarity to the human mineral constituent, leading to applications in the tissue engineering field [2], medical implants [3, 4], cell proliferation [5, 6] and in-vivo delivery of biomolecules (plasmid DNA [7, 8] or Protein [9]). HA-based nanostructures with size less than 100 nm exhibits enhanced resorbability [10] and bioactivity [5, 11], osteogenic activity [12-14], osteoblast adhesion [12] as well as a promotion of cell growth and inhibition of cell apoptosis [15], with respect to larger or micro-sized hydroxyapatite [16]. Various methods have been developed to prepare HA based nanoparticles such as dry methods [17], high temperature processes [18] and wet chemical methods [19-21]. The wet chemical methods are attractive because size and surface properties can be controlled at once. However this method involves the use of salts precursors and/or reducing agents [22] which can interfere with subsequent stabilization during the functionalization step or require multi-step procedures, i.e., long term aging and heat treatment steps. [23] Swain et al. reported crystalline hydroxyapatite nanoparticles with 60 nm mean size by co-precipitation technique combined with heat treatment in the range from 700°C to 1250°C. [24] Other groups obtained hydroxyapatite nanoparticles with a size around 100 nm by sol-gel approach and heat treatment steps (800°C), with crystallinity but different phases [23, 25]. Nowadays, Pulsed Laser Ablation in Liquid (PLAL) has gained a great deal of attention for simplicity of the procedure, which lets the synthesis of a wide range of nanoparticles, i.e., metallic [26-28], semiconductor [29-31], and ceramic [32]. Compared to chemical synthesis, PLAL technique appears to be the most flexible and promising technique, because of its many advantages for biomedical applications. (i) PLAL does not necessarily require any chemicals and does not necessarily produce waste: it is defined “green” method [33]; (ii) one step functionalization is easy, since the functional molecules can be simply added to the colloidal solution [34-36]; (iii) PLAL technique allows the production of NPs on the gram scale per hour [37]. In this work, we report on the formation of hydroxyapatite nanoparticles by ultra-fast laser ablation of hydroxyapatite target in deionized water and in aqueous solution of biomolecule. Laser ablation in deionized water results in the formation of large clusters of micrometers in size, i.e., ∼700 nm, composed by small nanoparticles while in biomolecules solution a starting process of clusters disaggregation is observed leading to the formation of nanoparticles with a mean size of ∼ 90 nm. Electron microscopy, dynamic light scattering and zeta potential measurements were performed, indicating that the presence of biomolecules in aqueous solution plays the role of stabilizing agents for HA-NPs. Further, the chemistry, crystallinity and chemical structure of the obtained colloidal solution is investigated by infrared spectroscopy, X-ray and Energy-dispersive diffraction.
2. Materials and Methods
2.1. Laser Synthesis of Hydroxyapatite Nanoparticles in Aqueous Solution
- Hydroxyapatite nanoparticles (HA-NPs) have been prepared by ultra-short laser ablation of hydroxyapatite bulk (HA) in deionized water and in aqueous solution of protein. Bovine Serum Albumin (BSA) protein is added to deionized water before the ablation at a concentration of 10-9 M. For each sample, the target was irradiated for 10 min at a fluency of 4 J cm-2. Laser ablation experiments were carried out using a picosecond Nd:YAG laser (continuum leopard) providing a pulse centered at 1064 nm with a maximum pulse energy of 115 mJ at a repetition rate of 20 Hz. The laser pulse energy was controlled with a variable attenuator. The laser beam was focused 1 cm below the target surface using a lens with a focal length of 25 cm. The hydroxyapatite target (CELLYARDTM pellet, PENTAX), in the form of a disc with a diameter of 5 mm and a thickness of 2 mm, was placed on the bottom of a quartz cuvette and immersed in 1 mL of solution. Before each experiment the target was washed with deionized water several times to remove the impurity from the surface. During laser ablation, the target was moved with a rotation system (T-cube DC Servo controller, Thor labs) to achieve uniform irradiation of the surface. Figure 1 shows a schematic of ultra-fast laser ablation of hydroxyapatite target placed in aqueous solution.
2.2. Characterisation
- Conventional Transmission Electron Microscopy (TEM) was performed with a JEOL Jem1011 microscope working at an acceleration voltage of 100 kV. Conventional TEM samples were prepared by dropping the colloidal solution directly onto a carbon-coated 300 mesh copper grids and allowing the aqueous solution to evaporate under room temperature and pressure. Energy-Dispersive X-Ray spectroscopy (EDX) characterization was performed on a High Resolution Scanning Electron Microscope (HRSEM) Jeol JSM-7500F equipped with a cold field emission gun using an Oxford X-Max 80 system with a silicon drift detector (SDD) having an 80 mm2 effective area. The analyses were done with an accelerating voltage of 10 kV and the spectra were acquired for 600 s live time. Standardless quantification was achieved with Aztec Energy EDX Software.X-Ray Diffraction (XRD) spectra were recorded using a Smartlab 9kW Rigaku diffractometer equipped with a copper rotating anode. The X-ray source was operated at 40kV and 150 mA. A Göbel mirror was used to obtain a parallel beam and to suppress Cu Kβ radiation (1.392 Å). The 2 theta/omega scan was performed with two radiations, Cu Kα1 (1.544 Å) and Cu Kα2 (1.541 Å), with a step of 0.05° (2theta). Specimens were prepared by drop-casting a solution of HA-NPs onto zero-background silicon wafers. The software PDXL by Rigaku was used for qualitative analysis.The morphology and zeta potential analysis of the laser-synthesized HA-NPs in deionized water and in BSA protein aqueous solution were performed using a Zetasizer Nano ZS (Malvern Instruments). Each solution was injected into disposal quartz cuvette for the evaluation of particles mean hydrodynamic size and into semi-micro disposable quartz electrophoretic cell for the stability evaluation. The Smoluchowski model was used to calculate the zeta potential value. All data represent the average of triplicate measurements of the samples prepared in three different procedures.Optical absorption spectra were recorded in quartz cuvette using a Cary 6000 UV-VIS-NIR double beam spectrophotometer. Fourier Transform Infrared (FT-IR) spectroscopy analysis were performed using the Bruker Vertex 80V infrared spectrometer in the range (600-4000) cm-1. Samples were prepared by drop-casting centrifuged solutions of HA-NPs on a CaF2 substrate and by drying them overnight in vacuum chamber.
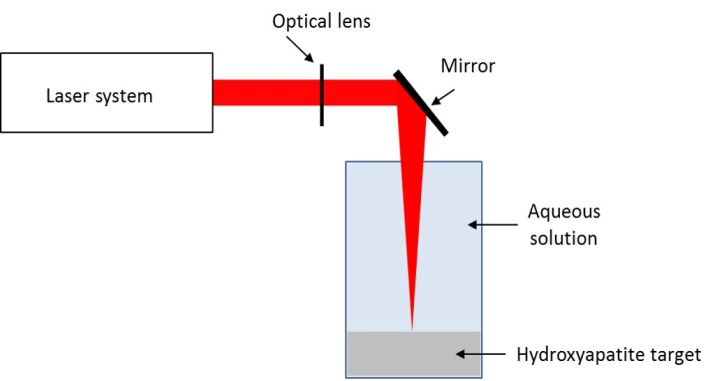 | Figure 1. Schematic of ultra-fast laser ablation of hydroxyapatite target placed in aqueous solution |
3. Results and Discussions
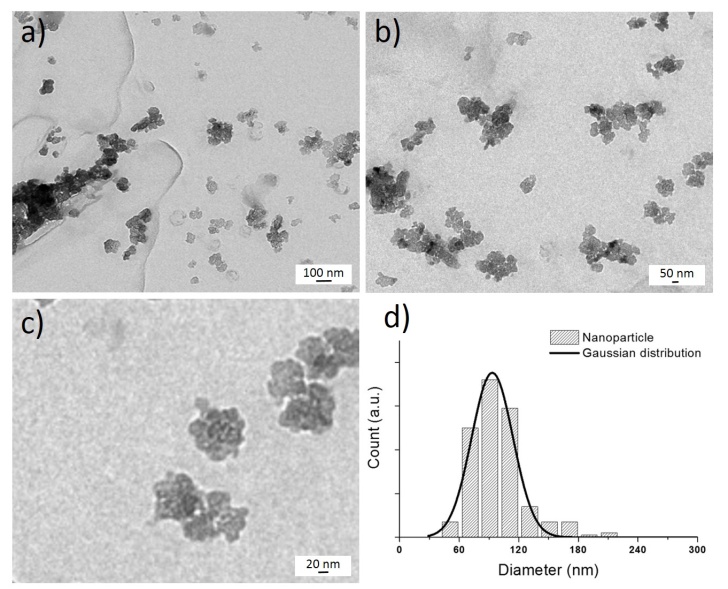
- HA-NPs colloidal solution were prepared by ultra-fast laser ablation of HA target in deionized water. Figure 2a shows the as-synthesized HA-NPs in water displaying the formation of HA aggregates of micrometers in size with a not well defined shape. The formed HA clusters are composed of small HA-NPs with size less than 50 nm, as shown in Figure 2b. The formation mechanism starts a few tens of picoseconds after the laser ablation and the plasma lasts for tens of nanoseconds. In the case of the ablation with nanosecond pulses, there is temporal overlap between the pulses and the laser-generated plasma, i.e., the ejected material and the heating related ablation can also occur in a region larger than the irradiated area. This temporal overlap produces an expanding plume composed by a mixture of ionized atoms, clusters and larger fragments, leading to the formation of large HA-NPs. [32] On the contrary, in the case of laser ablation with ultra-fast laser pulses, i.e., picosecond, there is no temporal overlap with the ablated material; the plasma evolves without any other heating, and radiation process resulting in the formation of small HA-NPs. Since the temperature in the laser-generated plasma is locally high (thousands of Kelvin), the nucleation and crystallization occur simultaneously allowing the formation of NPs nanostructures, while their agglomeration is the result of the low stability of the unconjugated HA-NPs, as shown in Figure 4.
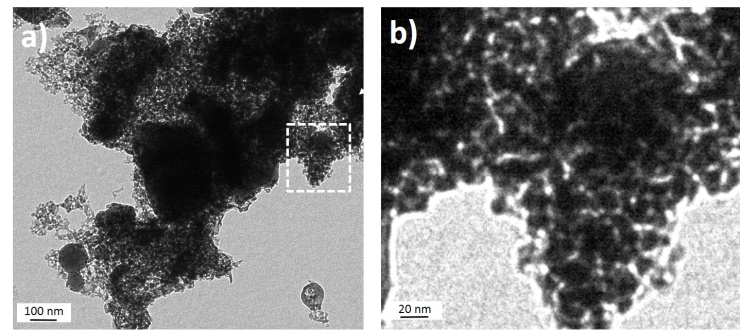 | Figure 2. a) TEM image of HA-NPs solution obtained by infrared picosecond laser ablation of HA target in deionized water. b) TEM magnification of the selected area, i.e., white dashed line |
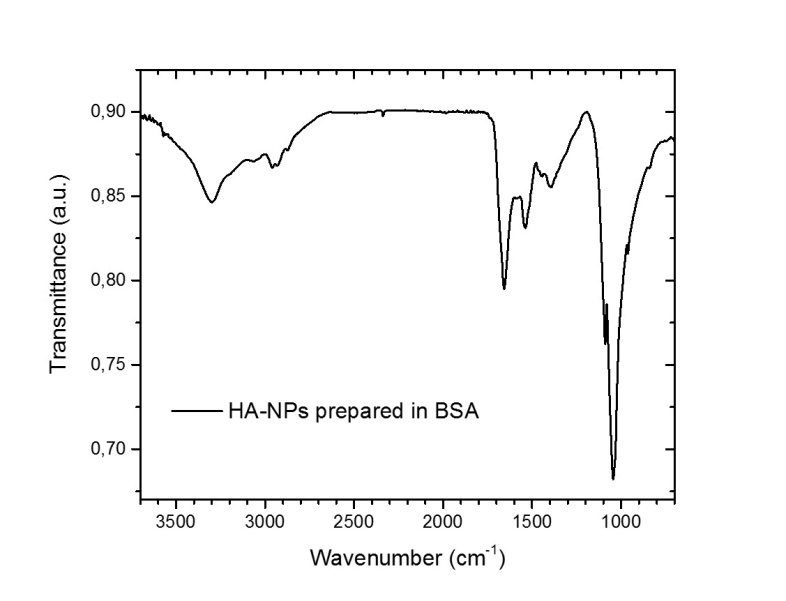 | Figure 7. FT-IR spectra of HA-NPs colloidal solution synthesized in 10-9 M BSA protein aqueous solution |
4. Conclusions
- We have reported on the synthesis and characterization of hydroxyapatite nanoparticles via ultra-fast laser ablation of hydroxyapatite target placed in deionized water and in aqueous solution of Serum Albumin protein. Electron microscopy, dynamic light scattering and zeta potential analysis showed that the synthesized HA-NPs in protein aqueous solution displayed HA nanoparticles with smaller size and less agglomerated compared to HA synthesized in deionized water. The Serum Albumin protein in solution acts as an in-situ size-regulating agent. Infrared spectroscopy, X-ray and Energy-dispersive diffraction indicated similar chemical and compositional structure to hydroxyapatite ceramic bulk, while the protein integrity after the laser irradiation was assessed by optical spectroscopy. Our synthesis approach overcomes conventional solution routes by avoiding the use of hazardous organic and/or unhealthy organic solvents and temperature treatments. Further advantages of being inexpensive, clean and easily scalable for biomaterials industry, open exciting possibilities to generate a wide range of HA-NPs, promising as versatile bioactive tool for in-vivo applications.
ACKNOWLEDGEMENTS
- The authors acknowledge Hamburg Centre for Ultrafast Imaging and Istituto Italiano di Tecnologia for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML