-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2018; 8(1): 11-24
doi:10.5923/j.nn.20180801.03

Recent Progress in Biomedical Applications of Nanodiamonds
Santos-Adriana Martel-Estrada
Instituto de Arquitectura, Diseño y Arte, Universidad Autónoma de Ciudad Juárez, Ciudad Juárez, México
Correspondence to: Santos-Adriana Martel-Estrada, Instituto de Arquitectura, Diseño y Arte, Universidad Autónoma de Ciudad Juárez, Ciudad Juárez, México.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Carbon based nanomaterials have unique advantages in several areas due to its electronic, optical, thermal, and mechanical properties. In these terms, nanodiamonds have attracted considerable attention in recent years in different research areas such as biological sensing, medical therapy, fluorescent markers, enzyme immobilization and so on. Nanodiamond (ND) is a new member of carbon nanoparticle family with a truncated octahedral architecture that showed superior characteristics of diamond. This review is focus on synthesis methods, dispersion, functionalization, medical applications and toxicity of nanodiamonds.
Keywords: Nanodiamonds, Polymers, Composites, Biomaterials
Cite this paper: Santos-Adriana Martel-Estrada, Recent Progress in Biomedical Applications of Nanodiamonds, Nanoscience and Nanotechnology, Vol. 8 No. 1, 2018, pp. 11-24. doi: 10.5923/j.nn.20180801.03.
Article Outline
1. Introduction
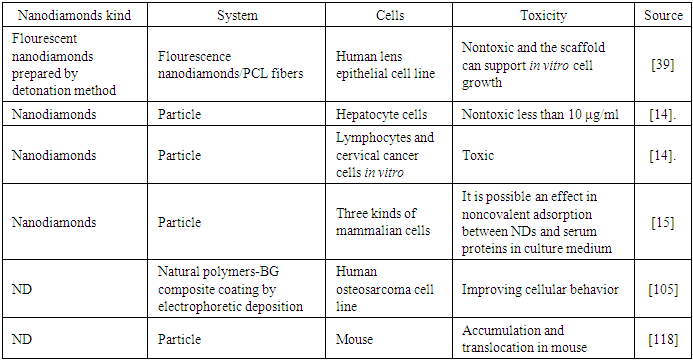
- Carbon based nanomaterials have unique advantages in several areas due to its electronic, optical, thermal, and mechanical properties [1]. Fullerenes, carbon natubes (CNT), carbon nanohorns, carbon nanodots, nanodiamonds (ND), and graphene (Figure 1) have attracted considerable attention in recent years [1]. Nevertheless, it is important to mention that while graphite is the stable carbon form at ambient conditions, diamond is metastable [2].
 | Figure 1. Carbon based nanomaterials [3-6] |
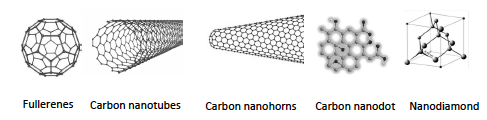
 | Figure 2. Main applications of NDs and SEM micrograph of commercial nanodiamonds purchased to Sigma-Aldrich |
2. Synthesis Methods
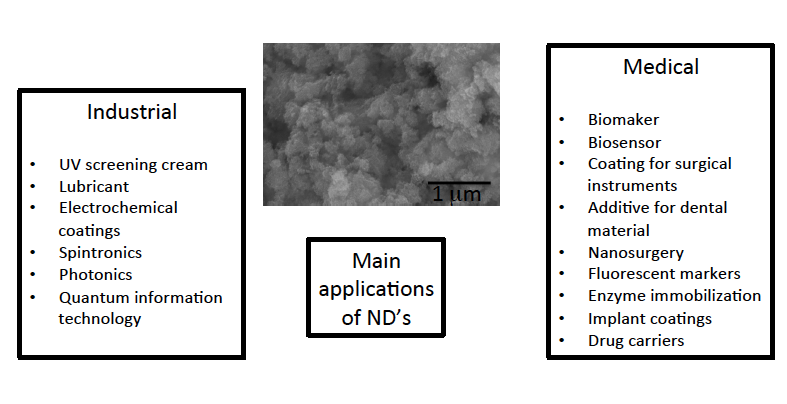
- A large variety of methods have been developed for synthesis of NDs, such as direct synthesis at ultrahigh pressures and temperatures, electron and ion beam techniques, chemical deposition of a carbon containing vapor at high temperatures and pressures, electrochemical anodic deposition, and detonation synthesis (Figure 3) [58].
 | Figure 3. Production methods of nanodiamonds |
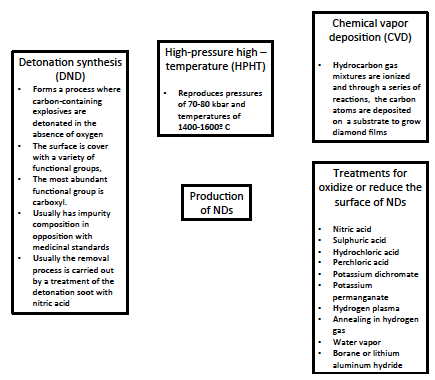
3. Detonation Nanodiamonds
- Detonation nanodiamonds (DND) were discovered in the 1960s. The interest in them rapidly grew over time due to their high potential for different applications [32, 63]. Through this preparation method nanoparticles maintain most of the physical properties of bulk diamond and exhibit a rich surface chemistry [62]. The main disadvantage of DNDs is the tendency to aggregate. At elevated temperature, starting from the outer layers, DNDs transform into concentric shells of graphitic sp2C and eventually resembles and onion. These onion ND particles exhibit layers of graphitic shells based on the degree of onionization [64]. DNDs are relatively low cost material of around $5-10 per gram [65]. They contain 80-88% of carbon in diamond phase, oxygen 10%, hydrogen 0.5-1.5%, nitrogen 2-3%, and incombustible residue of oxides, carbides or salts (0.5-8.0%) [50]. It has been reported that NDs are formed by a structure of mainly three layers [22, 66]: a) A diamond core 4-6nm (70-90% of all ND carbon atoms)b) A surface containing carbon atoms and different functional groups.c) A translational carbon shell around the core made from X-ray amorphous structures with a width of 0.4-1-0 nm. NDs produced by this method have a negative oxygen balance in a nonoxidative medium. Then, the diamond fraction is contained in a carbon stock [55].DND are considered suitable candidate for drug delivery. They contains primarily –OH, -CH3, -CH2 and –C=O groups. They are produced by the detonation of explosives such as trinitrotolueno (TNT) and hexogen (RDX) [67]. NDs are optically transparent and capable of fluorescing from point defects. The carbon surface of NDs is covered by a variety of oxygen functional groups which serves as the foundation for covalently attaching of bioactive molecules or other functional groups [9]. So, the surface can be functionalized with radioactive labels and compounds with biological activity [68]. Also NDs are photoluminescent, an important property in most applications [53], due that fluorescent labeling capacities can be used for both diagnostic and therapeutic purposes [45].
4. Fluorescent NDs
- NDs have excellent chemical stability, good biocompatibility and can be bonded due to the abundant surface functional groups [69]. They are biocompatible and have extraordinary optical properties include photostability, absence of photoblinking, fluorescent -vacancy (NV) centers within NDs that give a broad emission peak (maximum around 680 nm) [41], emitting in far-red/near infrared, perfectly adapted for biological labeling [16]. An NV center comprises a pair of substitutional nitrogen atom and lattice vacancy on the nanoparticle. These centers has two charge states: NV0, a neutral species and NV, a negatively charged species [43]. Fluorescent nanodiamond (FND) has a center that can emit bright far-red fluorescence at 700 nm wavelenght when excited by Green-yellow light and nearly 70 % of the emission lies in the near infrared window of biological tissue [70]. It has been reported that the first NV FND was prepared in laboratory by Beveratos using HPHT [71, 72]. Using the same method, fluorescent diamond nanocrystals (10nm diameter) were prepared for life science applications with fluorescent intensity and spin decoherence time depending on the nature of the post-milling chemical treatment [72]. FNDs have been produced in mass based on direct irradiation of NDs in aqueous colloidal solution with an homogenous distribution [38]. When the FNDs are carboxylated with oxiding acid, it can be produced carboxylated FND-COOH that can react with biomolecules to form covalent amide bonds [73].Frequent elements introduced in diamond are nitrogen, silicon, nickel, phosphorus, boron, and cobalt [74]. Silicon diamond is an ideal photon source fluorescence [49, 74]. Chromium-based and niquel-based single photon emitters are attractive due to their room temperature narrow bandwidth emission [49]. FNDs uses in biomedical imaging include diamond with nitrogen vacancy sites, extrinsic dyes and blue fluorescence [56, 75]. FNDs are considered biocompatible luminescent probes with exceptional optical properties for bioimaging and a center photostable showing no photobleaching or photoblinking [76, 77]. The fluorescence lifetime of NV center is 10 ns, so it is longer than lifetime of auto fluorescence, that it is 4 ns [76]. Also, they have been studied as targeted probes for long-term imaging and single particle tracking in vivo. They have photostability with photobleaching and bright-red fluorescence [2, 78]. Furthermore, it was shown that hydrogenated NDs are candidates as radiosensitizing agent associated with antisense molecular therapy [8].
5. Functionalization of NDs
- Applications of most of nanocarbons, including NDs have been limited because of inherent no compatibility with solvents, polymers and other matrices. The surface of NDs contains primarily -OH, -CH3, -CH2, CO2 and –C=O groups [68]. So, several methods to develop surface modification for NDs have been studied. The methods include mechanical disruption of the bundles, non-covalent and covalent methods, such as conventional chemical techniques (milling, sonication and refluxing). Many of these reactions required to be carried out over long periods of time. For example, for carboxylation, the reaction mixture was typically fluxed with strong acids of oxidizing agent for 10-50 h. Also, it has been proposed functionalization by microwave assisted process [79]. The ND particle surface can be functionalized with a large number of surface ionogenic groups (ether — C – O – C, peroxide — C – O – O –, carbonyl — C_O, and hydroxyl-type C – O – H bonding, etc.) as well as hydrocarbon fragments. The surface can also be modified with biologically active molecules by adsorption, covalent or non-covalent chemical immobilization [80]. If it is used a treatment with strong acids, it could be produced a formation of COOH groups on the surface which can react with alcohols and amines derivatives [18]. Another approach consist in reducing all oxygen-containing surface groups to OH functions with borane, then allowing the grafting of variety of silanes of long alkyl chains. Others procedures are halogenations and cold plasma functionalization and microwave plasma chemical vapor deposition [81]. Oxidation or hydrogenation of NDs produces carbon-hydrogen bond on the particle surface and the formation of positive zeta potential in colloidal solution. On the other hand, when NDs have groups containing oxygen on the surface it is produced a negative zeta potential of oxidized particles in colloidal solution [67]. The functional groups that it has been produced in NDs include: amines, silanes, butyl, hexyl, aminoacid, carboxyl, aryl, azid, benzoquinone, thymidine, cyanide, fluorine, alkyl, methyl, perfluorootyl ester, aliphatic, chlorocarbonyl, chlorine, amino, glycine, ether, phenyl, halides, and thiols. On the other hand, some biological moieties have been covalently bonded to nanodiamonds using amide bonds such as transferrin, green fluourescent protein, bovine serum albumin, porcin trypsin, growth hormone, mitochondria, actin, paclitaxel, DNA, biotin, folic acid, amino acid, etc. (Figure 4)
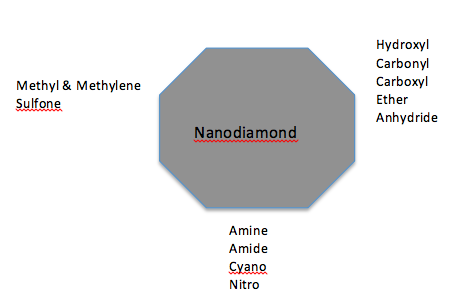 | Figure 4. Common functional groups on the nanodiamond surface [56] |
6. ND Dispersion
- NDs increase the initial elasticity modulus and breaking strength of composite materials [22]. Although, NDs tend to form aggregates, this can be used for a more effective treatment in vivo, in order to provide a better and a constant release of the drug from within the aggregates or means limitations in its application in biomedical area [46]. This strategy could decrease damage to remote healthy cells and reduce side-effects [37]. Also, NDs have high affinity in its surface with proteins [41]. Although, ND composites have poor dispersion and show agglomeration [88], it is a nanoparticle that can be made monodispersed with functionalizable surfaces [64]. Nevertheless, it could be found only one research that reports a stable water suspension with particles less than 100 nm in size [10]. Other researchers report that 75-80 % wt of primary aggregated NDs particles can be converted into nanoparticles, 10-20 nm in size by chemical modification [18]. For these reasons, usually strong aggregates are dispersed using grinding, milling with salts, sonication with the addition of salt and the addition of surfactant [56]. Then, in order to use NDs in biomedical area, it is needed a stable dispersion of particles in aqueous solution [56].NDs are highly stable in water when are coated by polymers produced from living polymerization, and in acidic and basic conditions, even in 1M NaCl and ethanol [41]. Nevertheless, DNDs have shown instability in hydrosolutions forming aggregates even when they are thawing of ice. So, some efforts have been made to solve it modifying its surface [89]. Moreover, salt and sugar with pH adjustment can produce stable aqueous ND colloidal solution [90]. Amine functional groups could be successfully grafted on DNDs by ball milling technique using ammonium bicarbonate or salt as a milling medium [19]. It is possible to control the zeta potential depending of the kind of ND used. For example, carboxylated NDs exhibit a negative zeta potential when dispersed in water at a pH>5. On the other hand, surface graphitization lead a positive zeta potential in water [8]. ND surface charge allowed the electrostatic loading of biomolecules such as siRNA [8, 75] and plant metabolites [8, 75].In addition, other research observed and characterized the influence of salts and proteins in the aggregation process and suggested strategies to improve the dispersion of particles for biological applications. The authors first suspended the particles in fetal bovine serum and then diluted in DMEM [91]. Furthermore, other studies purposed modified NDs by explosion synthesis that showed high colloidal stability in water and glucose solutions when used to binding lysozyme [92].
7. Medical applications
- Advances in nanomedicine such as imaging and diagnosis, drug delivery and gene therapy have demonstrated the benefits of nanoparticles therapy [93]. Nanoparticle-based nanoscience have great promise in medical applications, such as drug and gene delivery [94]. NDs were used in biomedical applications such as drug delivery, biomedical imaging and biosensors, due to their small size (2–10 nm), surface structure, and inertness (Figure 5) [95]. Nevertheless, until today, it is necessary to improve methods of drug delivery to maximize therapeutic effects while decreasing associated complications due to nonspecific or over-elution.
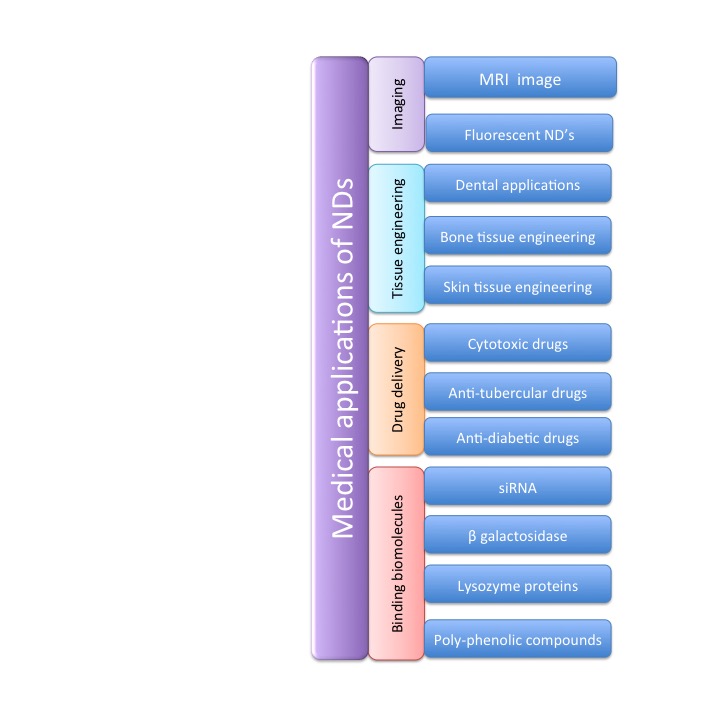 | Figure 5. Medical applications of nanodiamonds |
7.1. Use of NDs for Imaging
- The potential applications of NDs as nanoreinforcements in polymeric matrices have been limited due to the difficulties associated with their dispersion and aggregation during processing and the poor interfacial interaction with polymers [47]. For biomedical imaging, NDs should remain separate from one another to facilitate the attachment to relevant molecules [37].The use of coordinated water molecules on the surface of nanodiamonds make these particles a promising platform for MRI contrast agent, due that strong electrostatic potential on the nanodiamond cause a surface-mediated attraction toward water molecules creating a nanophase of water on the solvent interface. This method was used to monitoring solid hepatic tumors [98].Furthermore, as it was discussed before, NDs present nitrogen-vacancy centers that have intrinsic fluorescence properties so they are interesting tools for imaging and diagnostics [1]. Moreover, NDs have a refractive index higher than cytoplasm, so they show a strong light scattering signal that permit clearly distinguish them in cell by optical microscopy [75].In other research, FNDs for biological imaging were produced by ultrafast photo-initiated RAFT polymerization. The nanoparticles were functionalized with poly(2-methacryloyloxyethyl phosphorylcholine). During the experimental procedure, the cytotoxicity test showed that the composites were nontoxic [99]. Also, NDs have attracted attention in particle tracking, such as HeLa cells and gonads of C. elegans [100]. Fu, et al. conjugated fluorescence nanodiamonds with BnK CT, a chlorotoxin like peptide isolated from the venom of Buthus mantensii Karsch. This conjugated was used to visualize and confirm glioma cells by confocal flurescence assay [101].
7.2. Tissue Engineering Applications of NDs
- Because of NDs are inert, non-toxic and biocompatible [56], they could be used to reinforce polymers for bone surgery and tissue engineering [11]. Some researchers have suggested that these nanoparticles have bioactive and antibacterial properties useful for dental implants [102]. Lee, et al., developed a composite of ND with gutta percha, a typical dental filler material for endodontic therapies. Their results showed an improvement in mechanical properties and bacterial growth inhibition when the composite was functionalized with amoxicillin [103].On the other hand, it has been shown that particles uptaken by cells are minimally cytotoxic and biocompatible, which means that they do not affect mitochondrial function or ATP production at the cellular level [56]. In this sense, it was studied that NDs are able to enter directly in cell nucleous and they could be thrown out the cytoplasm by exocytosis [75]. Nevertheless, other researches showed that ND did not influence cell viability, cell membrane or intracellular oxidative stress and their cellular influences are less than other nanocarbons [75, 104].Because of their size one can expect the elimination of the particles by kidney [45]. Nevertheless, although some publications consider NDs biocompatible and no toxic, other works suggest that biocompatibility should not be overgeneralized [76]. Some studies showed that NDs accumulate in the lung, liver, spleen, and kidney in a day and its clearance lasted over 10 days [56].In spite of these results, NDs have been used as fillers of biocomposite scaffolds that promote cellular attachment, differentiation and proliferation with bioactive glass (BG) particles produced by the method of electrophoretic deposition. In that study, it was used alginate as a matrix and the in vitro bioactivity assay showed the formation of hydroxyapatite on the surface of the composite films [105].On the other hand, in bone tissue engineering were obtained nanocomposites with properties close to the human cortical bone using fluorescent nanodiamonds with poly(l-lactic acid) (PLLA) [106]. Poly(vinylidene fluoride) with NDs was evaluated morphological, structural, optical, thermal and electrically. Therefore, it was evaluated the cell culture viability with pre-osteoblast cells. During this study, it was proven that porous samples were more appropriate than films for proliferation [107]. Also, NDs were used conjugated with alendronate for bone target delivery. It was evaluated the ALP activity and specific uptake for MC3T3-E1 of the drug system. The results showed high affinity of alendronate/NDs with HAp, so it could be potentially used for osteoporosis treatment [108].Other research showed that poly(L-lactic acid)-FNDs composite for bone engineering, reached uniform dispersion and good affinity between the matrix and the nanoparticle. Also, the composite was nontoxic to murine osteoblasts [106].In skin tissue engineering, it was used irradiated NDs and polycaprolactone, creating a superior cell-interfacing material with a less hydrophobicity. This substrate was capable of in vivo detection [109].Cai, et al., evaluated the effect to add NDs to Poly(Lactic Acid) in nanofiber membranes. The fiber diameter decreased as ND content increased. Also, the nanoparticles improved the thermal and mechanical properties of the composite, so they have potential applications in biomedical engineering [110]. Furthermore, poly(lactic-co-glycolic acid) loaded with NDs phospholipid showed high mechanical properties, good in vitro and in vivo biocompatibility NDs. The composite was evaluated for in vitro proliferation and differentiation of human fetal osteoblastic cells. It was found that the material could promote osseointegration [111].
7.3. Use of NDs for Drug Delivery
- Nanoparticles are immensely used in cancer management due to their versatile attractive features such as small size, stability, inertness, increased surface-to-volume ratio, highly tunable optical properties, and suitability for surface modification [6]. Several researches show the successful conjugation of nanoparticles to therapeutic drugs, imaging agents, polymers and cancer targeting ligands [46-48]. Nanoparticles could be customized for delivering the chemotherapeutic or cancer diagnostic agents selectively to the cancer cells [112]. For example, chemotherapeutic agent could be conjugated to ligand target nanoparticles allowing the discrimination of cancer cells from the fast growing healthy cells of the body. Then the cytotoxic drugs could be delivered only to the cancer cells [112]. Also, they could be functionalized with doxorubicin to use as an inhibitor of lung metastasis in mice [75] or with ciproten and quercetin for cancer treatment [75].Anticancer agents can functionalize the surface of nanomaterials via chemical activation of surface groups or by non-covalent binding [68]. So, previously a research group developed a polysaccharide sodium alginate functionalized NDs for chemotherapeutic drug delivery of cis-diamminedichloro platinum (II) [12].ND drug delivery systems could be obtained by adsorption of a drug on the surface of NDs and by covalent binding of a drug with surface functional groups [18]. So, NDs have been used too as a adsorbent of antidiabetic and antitubercular drugs [18]. Also, the particle could be used for separation and purification of proteins, such as immunoglobulins of human blood [113] or other components of human blood serum [114]. Furthermore, NDs were functionalized with N-O-carboxymethyl chitosan as an ideal candidate for drug delivery [7]. Modification of the NDs surface with biologically active compounds and drugs offers a potential application of it with anticancer therapeutic agent such as doxorubicin, daunorubicin or cisplatin [68]. They have been tested as a potential carries of myramistin showing easy drug adsorption and drug release under physiological conditions only [68]. Moreover, it has been shown that ND at 25 μg/ml concentration did not promote apoptosis, an inflammatory response, or inhibited proliferation at the level of the transcriptional response [56].Wang, et al., [115] used a transferrin-doxorubicin complex with carboxylated ND. Their results showed that the complex could deliver the drug inside living cells via a clathrin-dependent and transferrin receptor-mediate endocytosis pathway. Also, they found that the complex reduced the volume of a tumor more than DOX-treated.On the other hand, in order to used NDs as a drug delivery systems it is required colloidal stability in physiological medium [68]. The stability could be increased using surfactants [68]. Previously, NDs were functionalized with a beta cyclodextrin and hyperbranched polyglycerol polymers to explore their biomedical applications, improving dispersion in water and biocompatibility. Also, they showed that DOX could be absorbed by the composite with high efficiency [116]. Other research group showed that it could be produced NDs that includes amino acid, peptides, oligonucleotides, sugars, etc., for drug delivery [117].Furthermore, inhibition of angiogenesis with VEGD inhibitors could be an important and effective strategy for the treatment of cancer metastasis. Sorafenib, is an efficient anti-proliferative and antiangiogenic drug that block Raf signaling and VEGF. Nevertheless, sorafenib is almost insoluble in water or buffered solution at certain pH values (from 1.2 to pH 7.4) and the oral bioavailability is extremely low (about 8.43 %) which greatly restricts its therapeutic efficacy on cancer metastasis. So, the particles were used in a lipid-coated ND as a drug delivery platform, improving the oral bioavailability of lipophilic drugs [13].NDs are particles among 5 and 100 nm that could be functionalized and conjugated with biomolecules [75, 80], so they have been used as a vehicle for the delivery of insuline [93] and for easy biomolecule immobilization [118]. Furthermore, NDs have antibacterial activity that it is influenced by their surface chemistry and size [119]. Nevertheless, biomedical applications of NDs commonly require that NDs be delivered and used in aqueous solutions [120].For medical applications such as biomolecule immobilizations, it is highly desirable to have diamond in a thin fiber or film form [40]. Mainly the chemical vapor deposition is the technique that is used for preparing biocompatible diamonds films [40].
7.4. NDs for Binding Proteins and Lipids
- It is possible to modify NDs surface to improve the interaction between the particle and its environment [51]. Hydrogenated NDs are stable in aqueous solutions, so these cationic particles were used to efficiently deliver siRNA to human cells [16]. In addition, cellular toxicity of ND-pani nanoparticles and films was evaluated using embryonic kidney cells. The study showed that the toxicity was dependent of the NDs concentration [121].It was shown that NDs support neural differentiation over glial differentiation with potential results to improve tissue integration and prevent the glial scar formation in implants [59]. Also, the particles were evaluated for enzyme immobilization, such as β galactosidase with broad applications in lactose-free dairy products [9].Other medical applications includes NDs functionalized with lysozyme proteins showing antibacterial activity [86]. Also, it was shown that NDs inhibit EWS/FLI-1 cells expression in culture [45]. NDs were used to inhibit the lung metastasis of breast cancer, inducing tumor cell apoptosis with reduced systemic toxicity [48]. Furthermore, nanocomposites elaborated with carboxylated NDs and cellulose have been evaluated as a wound dressing showing potential use in skin engineering [69].Recently, Cheng, et al., [122] functionalized NDs with vitamin E TPGS to improve oral absorption of curcumin, a lipophilic poly-phenolic compound that exhibits biological activities such as anti-oxidant, anti-inflammatory and anti-cancer properties. Their results showed that the absorption of the nanocomplexes was significantly improved in the GI tract biodistribution.NDs have high affinity in its surface with proteins. Then, proteins can remain attached with them, even after washing. Mainly, protein adsorption includes electrostatic, hydrophilic and hydrophobic interactions, hydrogen bonding, and van der Waals forces [41]. Due to this high affinity for proteins, they were used to find biomarkers of the secreted proteins from carbapenemase-producing A. baummannii. The spread of carbapenem-resistant Acinetobacter baumannii is a challenge for optimization of antibiotic therapies and outbreak prevention, so this method could be used for the detection of the bacteria [123].Li, et al., evaluated the receptor-mediated endocytosis of fluorescent NDs linked with transferrin. Their results showed that the crosslinking of the nanoparticles with proteins promote better stability and efficiency for cellular uptake [124]. Furthermore, NDs tend to precipitate at physiological ionic strength, and proteins are readily adsorbed on the exposed surface [41]. So, the apoobelin protein was separated and purifying using NDs in a chromatography [83]. On the other hand, it has been proved that ND can adsorb peptides [125] and amplify signals and detection of miRNA-21 [126].Other research used as a suspension for relieves the state of oncological patients, decreasing probably the level of intoxication. The particle was used to correct protein and lipid peroxide oxidation processes associated with malignant tumor growth [65].The particle has been used in advanced composites for biomedical purposes. For instance, nanofibers with NDs and polycaprolactone were produced by electrospinning using acetone as a solvent exhibiting bright flourescence [39]. It has been reported polymer nanoparticles from poly(vinylpyrrolidone) (PVP) using ultrasonication with a uniform dispersion of the NDs[88] and NDs with iodine-125 labeled immunoglobulin and bovine serum albumin and Rabbit Anti-Mouse Antibody by covalent immobilization [127].Also, it is possible to modify NDs with a positively charged polymers but those kind of materials do not ensure shielding from protein and cell interactions. Nevertheless, they can serve as a platform for nucleic acid complexation and as gene deliver vectors [41].
7.5. Some Considerations about Toxicity of Nanodiamonds
- NDs, like several particles of nanometric dimensions, have been subject to continuous evaluation of their toxicity (Table 1). Depending on the type of application, a certain degree will be desirable, or maybe a totally undesirable characteristic. Although, MTT (3-(4,5-dumethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and adenosine triphosphate (ATP) assays have shown that NDs are non-toxic to a variety of cell types [105], it was found significant lung and systemic toxicity of NDs after intratracheal instillation suggesting further toxicological studies [128]. So, inevitably, the biological behavior and potential toxicity of nanodiamonds are topics of interest [118].
|
8. Conclusions
- During the last decades, nanodiamonds have been studied due to their superior physical and chemical properties of versatile functionalization, such as large surface area, high adsorption capacity and good biocompatibility. These particles have been used in many research areas such as biological sensing, medical therapy, composite materials, lubricants, fluorescent markers, electrochemical coatings, enzyme immobilization and implant coatings. NDs have shown biocompatibility for many biomedical applications. Nevertheless, due that it not exist an agreement about its toxicity, more experiments could be developed in order to elucidate the potential risks in different tissues.In spite of its unique characteristics, it has not been explored enough the possibility to improve its compatibility with different solvent and polymers. Furthermore, until today, it is a challenge to disperse NDs in a solution for an effective application in biomedical area, so future studies should explore particle systems for this purpose.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML