| [1] | A. K. Geim, Graphene: status and prospects, Science, 2009, Vol. 324, no. 5934, PP. 1530–1534. |
| [2] | M. Pumera, Graphene in biosensing, Materials today, 2011, Vol. 14, PP. 308–315. |
| [3] | J. Liu, L. Cui and D. Losic, Graphene and graphene oxide as new nanocarriers for drug delivery applications, 2013, Vol. 9, PP. 9243-9257. |
| [4] | D. Cohen-Tanugi and J. C. Grossman, Water Desalination across Nanoporous Graphene, Nano Letter, 2012, Vol. 12, PP. 3602-3608. |
| [5] | A. A. Moosa, A. Ramazani, and M. N. Ibrahim, Effects of carbon-nanotubes on the mechanical and electrical properties of epoxy nanocomposites, American Journal of Materials Science, 2016, Vol. 6, No.6 pp. 157-165. |
| [6] | A. A. Moosa, F. Kubba, M. Raad and A. Ramazani, Mechanical and thermal properties of graphene nanoplates and functionalized carbon-nanotubes hybrid epoxy nanocomposites, American Journal of Materials Science, 2016, Vol. 6, No. 5 pp. 125-134. |
| [7] | A. A. Moosa, A. Ramazani, F. Kubba, M. Raad, Synergetic effects of graphene and nonfunctionalized carbon nanotubes hybrid reinforced epoxy matrix on mechanical, thermal and wettability properties of nanocomposites, American Journal of Materials Science, 2017, Vol. 7, No. 1, PP. 1-11. |
| [8] | S. Thakur and N. Karak, Alternative methods and nature-based reagents for the reduction of graphene oxide: a review, Carbon, Vol. 94, 2015, PP. 224–242. |
| [9] | K. S. Novoselov, A. K.Geim, S. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Electric field effect in atomically thin carbon films, Science, 2004, Vol. 306, No. 5696, PP. 666–669. |
| [10] | A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M.S. Dresselhaus and J. Kong, Large Area, few-layer graphene films on arbitrary substrates by chemical vapor deposition, Nano Letter, 2009, Vol. 9, PP. 30–35. |
| [11] | S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen and R. S. Ruoff, Synthesis of graphene-based nano sheets via chemical reduction of exfoliated graphite oxide, Carbon, 2007, Vol. 45, PP. 1558–1565. |
| [12] | H. Huang, W. Chen, S. Chen and A. T. S. Wee, Bottom-up growth of epitaxial graphene on 6H-SiC(0001), ACS Nano, 2008, Vol. 2, PP. 2513–2518. |
| [13] | M. J. McAllister, J. L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. H. Alonso, D. L. Milius, R. Car, R. K. Prudhomme, and I. A. Aksay, Single sheet functionalized graphene by oxidation and thermal expansion of graphite, Chem. Mater., 2007, Vol. 19, PP. 4396–4404. |
| [14] | A. Ambrosi, and M. Pumera, Precise tuning of surface composition and electron‐transfer properties of graphene oxide films through electroreduction, Chemistry–A European Journal, 2013, Vol. 19, No. 15, PP. 4748-4753. |
| [15] | S. Park, R.S. Ruoff, Chemical methods for the production of graphenes, Nature Nanotechnology, 2009, Vol. 4, PP. 217–224. |
| [16] | N. Mohanty, A. Nagaraja, J. Armesto and V. Berry, High‐throughput, Ultrafast Synthesis of Solution‐dispersed Graphene via a Facile Hydride Chemistry, Small, 2010, Vol. 6, PP. 226–231. |
| [17] | J. Wang, E. C. Salihi, L. Siller, Green reduction of graphene oxide using alanine Materials Science and Engineering C, 2017, Vol. 72, PP. 1–6. |
| [18] | Y. Wang, Z. Shi, J. Yin, Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites, ACS Appl Mater Interfaces. 2011, Vol. 3, No (4), PP.1127-33. |
| [19] | O. Akhavan, E. Ghaderi, S. Aghayee, Y. Fereydooni and A. Talebia, The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy, Journal of Materials Chemistry, 2012, Vol. 22, PP.13773-13781. |
| [20] | J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Reduction of graphene oxide via L-ascorbic acid, Chemcal Communication, 2010, Vol. 46, PP. 1112–1114. |
| [21] | T. Kuila, S. Bose, P. Khanra, A.K. Mishra, N.H. Kim and J.H. Lee, A green approach for the reduction of graphene oxide by wild carrot root, Carbon, 2010, Vol. 50, PP. 914–921. |
| [22] | E. C. Salas, Z. Sun, A. Luttge and J.M. Tour, Reduction of graphene oxide via bacterial respiration, ACS Nano, 2010, Vol.4, PP. 4852–4856. |
| [23] | W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, Journal of the American Chemical Society, Vol. 80, No. 6, PP. 1339, 1958. |
| [24] | D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, Improved synthesis of graphene oxide, ACS Nano, 2010, Vol. 4, PP.4806–4814. |
| [25] | H. A. Becerril, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, Evaluation of solution-processed reduced graphene oxide films as transparent conductors, ACS Nano, 2008, Vol. 2, PP. 463–470. |
| [26] | S. Abdolhosseinzadeh, H. Asgharzadeh and H. S. Kim, Fast and fully-scalable synthesis of reduced graphene oxide, 2015, Vol. 5, P. 10160. |
| [27] | Y. Zhou, Q. Bao, L. A. L. Tang, Y. Zhong, and K. P. Loh, Hydrothermal dehydration for the “Green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties, Chemistry of Material, 2009, Vol. 21, PP. 2950-2956. |
| [28] | S. Hatamie, O. Akhavan, S. K. Sadrnezhaad, M. M. Ahadian, M. M. Shirolkar and H. Q. Wang, Curcumin reduced graphene oxide sheets and their effects on human breast cancer cells, Materials Science and Engineering, 2015, Vol. 55, PP. 482–489. |
| [29] | D. Suresh, Udayabhanu, M.A. Pavan Kumar, H. Nagabhushana and S.C. Sharma, Cinnamon supported facile green reduction of graphene oxide, its dye elimination and antioxidant activities, Materials Letters, 2015, Vol. 151, PP. 93–95. |
| [30] | M. Jana, S. Saha, P. Khanra, N. C. Murmu, S. K. Srivastava, T. Kuila and J. H. Lee, Bio-reduction of graphene oxide using drained water from soaked mung beans (Phaseolus aureus L.) and its application as energy storage electrode material, Materials Science and Engineering B, 2014, Vol. 186, PP. 33-40. |
| [31] | D. Suresh, P.C. Nethravathi, Udayabhanu, H. Nagabhushana and S.C. Sharma, Spinach assisted green reduction of graphene oxide and its antioxidant and dye absorption properties, Ceramics International, 2015, Vol. 41, PP. 4810–4813. |
| [32] | R. K. Gupta, Z. A. Alahmed, F. Yakuphanoglu, Graphene oxide based low cost battery, Materials Letters, 2013, PP. 75–77. |
| [33] | Z. Bo, X. Shuai, S. Mao, H. Yang, J. Qian, J. Chen, J. Yan and K. Cen, Green preparation of reduced graphene oxide for sensing and energy storage applications, Scientific Reports, 2014, Vol. 4, P. 4684. |
| [34] | X. Fan, W. Peng, Y. Li, X. Li, S. Wang, G. Zhang, and F. Zhang, Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation, Advanced Materials, 2008, Vol. 20, PP. 4490-4493. |
| [35] | A. K. Meena, G. K. Mishra, P. K. Rai, C. Rajagopal and P. N. Nagar, Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent, Journal of Hazardous Materials B, 2005, Vol. 122, PP. 161 – 170. |
| [36] | N. K. Mandal, Performance of low-cost bio adsorbents for the removal of metal ions, International Journal of Science and Research, 2014, Vol. 3, PP. 70-74. |




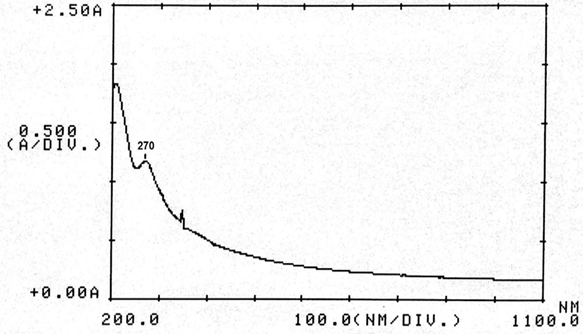
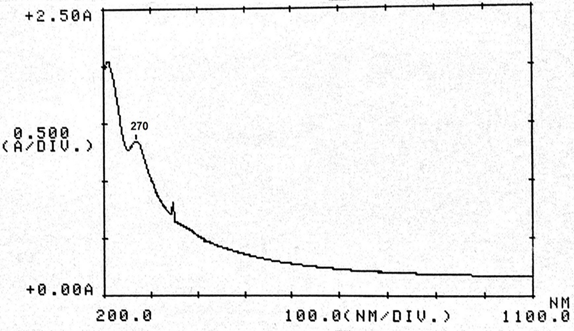
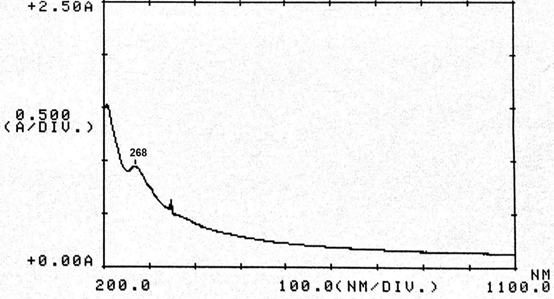
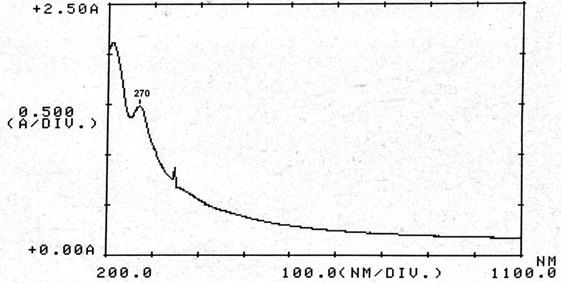
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML