-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2017; 7(1): 14-20
doi:10.5923/j.nn.20170701.04

Durable Multifunctional Properties on Polyester Fabric by Applying Nanocoating
Md. Mostafizur Rahman
College of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan, China
Correspondence to: Md. Mostafizur Rahman, College of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan, China.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work is done to prepare multifunctional fabric by coating with nano composite made of reduced graphene oxide and TiO2 on polyester fabric. The influence of the RGO coatings on polyester fabrics imparts properties such as light absorption, conductivity, electroactivity and photocatalytic properties. With the increased number of RGO layers the properties also developed. RGO treated polyester fabric was then treated with TiO2 to enhance the multifunctional properties of the fabric. The photo-catalytic properties of the fabrics were tested with Rhodamine B dye solutions. Photocatalytic efficiency increased with the number of RGO coatings, due to the increased light absorption, and better electrical properties. Moreover, the nanocomposite finished polyester fabric demonstrated proper antimicrobial properties and UV blocking activity.
Keywords: Polyester fabric, Graphene Oxide, TiO2, Multifunctional properties, Cationic Modification
Cite this paper: Md. Mostafizur Rahman, Durable Multifunctional Properties on Polyester Fabric by Applying Nanocoating, Nanoscience and Nanotechnology, Vol. 7 No. 1, 2017, pp. 14-20. doi: 10.5923/j.nn.20170701.04.
1. Introduction
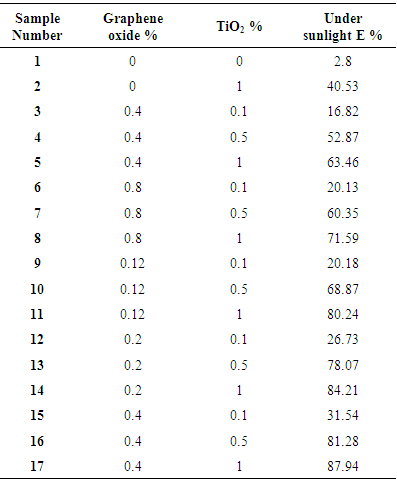
- Now a days functional fabrics with different kind of properties are being so much popular and an interesting topic to researchers. Various kinds of properties are being applied on textiles like photocatalytic, fire retardant, water proof, UV absorbent etc. An innovative strategy for functional finishing of textile fabrics is nano coating. Nowadays, the variety of nanomaterials used for textile finishing includes nano photocatalysts like TiO2, ZnO, SrTiO3 and ZrO2. Among them, nanoTiO2 applications has great promise due to its fascinating properties, such as optical and electronic properties, abilities to purify pollutions, chemical stability, and nontoxicity. Titanium dioxide photocatalyst has received significant interest in recent years due to its fascinating properties, such as optical and electronic properties, low cost, abilities to purify pollutions, long term stability and nontoxicity [1, 2]. Deposition of titanium dioxide nanoparticles on textiles provides new properties such as self-cleaning, super hydrophilic, antibacterial, UV-protection, flame retardancy, etc [3-10]. Graphene, one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed into a 2-D honeycomb crystal lattice, has become one of the most attractive materials due its unique chemical and physical properties as well as its diverse potential applications [11, 12]. Moreover, graphene oxide, an atomic sheet of graphite with a wide range of functional groups (epoxy, hydroxyl, and carboxyl), is one of the most important derivatives of graphene.One obvious way to exploit the properties of either graphene or nano titanium dioxide is to combine them. This allows the properties of each constituent to be conserved or modified. The poor solubility of graphene in water or polar organic solvents makes it difficult to deposit nanoparticles on its surface. The presence of oxygen containing functional groups in graphene oxide makes it as excellent supporters to anchor TiO2 nanocrystals in liquid phase [13].Graphene can serve as a conductor and electrons are transferred from the conduction band of TiO2 to graphene sheets, this difficulties the recombination of the pair electron/hole and increases the lifetime of the pair. Moreover, the efficiency of the system is increased by a greater generation of radicals. Other benefit of employing graphene and its derivatives is the great adsorption capacity of organic molecules, which allows a better contact between the pollutants and the catalytic surface area. Another plus that has been observed is an increased absorption in the spectral range due to an increased band-gap of TiO2 arising from the interaction with graphene derivatives. Several studies have focused on the employment of graphene and TiO2 in heterogeneous photocatalysis [14-18].In this paper, the combination of TiO2 and RGO to produce photocatalytic fabrics is studied. A Bovine serum albumin coating were applied to increase the fixation and homogeneity of the RGO coatings. GO was reduced to RGO and different number of RGO coatings was applied to improve the conductivity of the fabrics. Thereafter, TiO2 nanoparticles were deposited by immersion of the RGO-coated fabrics in a TiO2 solution. An electrochemical approach has been employed to study the influence of the conductivity and the electrochemical properties of the graphene coated fabrics on the photocatalytic properties of the fabrics. The photocatalytic properties of the fabrics were tested with the degradation of Rhodamine B solutions.
2. Experimental
- Materials: Bovine serum albumin (BSA), Commercial TiO2 nanoparticles, Polyester (PES) fabrics characteristics with surface den-sity, 100 g m-2; warp threads per cm, 60; weft threads per cm, 23. sulphuric acid (H2SO4) and potassiumchloride (KCl), K4Fe(CN)6 99%.Synthesis of Graphene Oxide: GO was synthesized from graphite powder by the modified Hummer’s (Hummers & Offeman, 1958) method in two distinct oxidation and filtering phases. During the oxidation phase, 5 g of graphite powder was added to 200 mL of concentrated H2SO4 in an ice bath with continuous stirring for 30 min. KMnO4 (25 g) was added slowly at temperature no higher than 10°C and was left stirring for next 30 min. Later, the mixture was allowed to react at 35°C for about 6 h with vigorous stirring. To stop the reaction, the temperature was dropped to 10°C with the use of ice, and 250 mL of D.I water was added very slowly. During the addition of water, the temperature was kept less than 55°C. Afterwards, 5 mL of H2O2 (30%) was added and then, the mixture was stirred for 30 more min. Then, this mixture was kept for 2 h followed by rinsing the supernatant during the filtering phase, with 0.5 L of 10% HCl and then 0.5 L water. At that time, 250 mL of water was added to the resulting product to form dispersion. The GO was bath sonicated for 30 min. Removal of unexfoliated GO sheets was done by centrifugation of the solution for 5 min at 10,000 rpm. Finally, dialysis of the solution was realized to remove the inorganic ions in the suspension.Application of GO on Polyester surface: GO presents negative charges so the deposition of GO on the fabrics is not possible in these conditions since electrostatic repulsion does not allow it. This is why bovine serum albumin(BSA) was employed as an intermediate coating that acquired positive charge and allowed the deposition of GO on the surface of the fabrics. It can attach both negative and positive charged surfaces depending on the pH. A 0.5% weight BSA solution was employed to coat fabrics [19]. Fabrics were put in contact with the BSA solution during 10 min to allow its adsorption, after this time fabrics were rinsed with water the remove the BSA’s excess. The pH of GO solution have to be 2.0. Fabric would be soaked in that solution for 30 min at 110°C, making it easier for GO to coated on fabric. At this stage thermal reduction of GO takes place. After this time, fabrics with RGO were dried under room conditions.Dispersion of TiO2 on the RGO fabrics: A solution of 5 g L-1 (500 mL) of TiO2 nanoparticles was prepared in water. An anionic surfactant was added (Setamol BL) (1 mL) to increase the fixation of TiO2 nanoparticles on the fabrics. The solution was stirred during 5 min. After this time, the RGO fabrics were put in contact and stirred with the solution during 2 min. After this, the fabrics were padded two times at 1 bar pressure and finally dried in an oven at 80°C.
3. Result and Discussion
- Photocatalytic activity: The photocatalytic activity of all samples was calculated by measuring the degradation of Rhodamine B (Rh-B) aqueous solutions (4 mg L-1) under UV light irradiation. Rh-B was selected for its good resistance to light degradation (the dye sensitization). Here, the polyester-RGO/TiO2 samples (5 cm × 5 cm) were dipped into a flask with 300 ml of Rh-B aqueous solution. Then it was stirred for 40 min in dark to reach the adsorption desorption equilibrium and then fitted in an UV simulation chamber. Then, the samples were irradiated with a 300 W UV lamp from a distance of around 20 cm. The average irradiance was around 40 W/m2.Absorbance of the Rh-B solution was monitored during 420 min (at time intervals of 40 min up to 120 min) and then at intervals of 60 min up to 420 using a spectrophotometer in the range of 300-700 nm. For this, some aliquots of Rh-B solution (3.5 mL) were taken out and then analyzed by monitoring the magnitude variation of its main absorption peak (around 564 nm). The rate of Rh-B consumed in a chemical reaction can be written as:
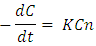 Where C is the concentration of the Rh-B aqueous solution, n is the kinetics order of the chemical reaction, and k is the rate constant of the photo degradation process.At low concentrations and for a specific time instant, the absorbance of the solution, At is related to the solution’s concentration through the Beer–Lambert law, that is, At= ε·l·Ct, where ε is the molar extinction coefficient, l is the light path length, and Ct is the solution concentration. For a first order kinetics reaction, the photo degradation efficiency,
Where C is the concentration of the Rh-B aqueous solution, n is the kinetics order of the chemical reaction, and k is the rate constant of the photo degradation process.At low concentrations and for a specific time instant, the absorbance of the solution, At is related to the solution’s concentration through the Beer–Lambert law, that is, At= ε·l·Ct, where ε is the molar extinction coefficient, l is the light path length, and Ct is the solution concentration. For a first order kinetics reaction, the photo degradation efficiency, 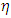 , of Rh-B can be calculated according to the following equation:
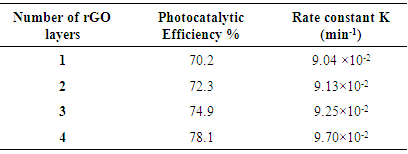
, of Rh-B can be calculated according to the following equation: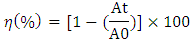 Where A0 is the absorbance at zero time. Therefore, the change of Rh-B concentration can be evaluated by measuring the change in the intensity of its main absorption peak.The photocatalytic activity of RGO and TiO2 coated fabrics was evaluated in terms of degradation of Rh-B under UV light irradiation.Fig.1 shows the control experiment where Rh-B solution is irradiated in the presence of polyester fabric in the same conditions applied to the catalytic fabrics. During the first 40 min a slight adsorption was observed. Thereafter, absorbance increased with the increasing due to the evaporation of part of the water due to the heat generated by the UV lamp. The final absorbance of the solution was higher than the initial one, this discards degradation of Rh-B by UV irradiation. Adsorption was also minimal as explained. Fig. 2 shows, for different irradiation times in the presence of polyester-RGO + TiO2 fabric, the evolution of the absorption spectra of Rh-B solutions.
Where A0 is the absorbance at zero time. Therefore, the change of Rh-B concentration can be evaluated by measuring the change in the intensity of its main absorption peak.The photocatalytic activity of RGO and TiO2 coated fabrics was evaluated in terms of degradation of Rh-B under UV light irradiation.Fig.1 shows the control experiment where Rh-B solution is irradiated in the presence of polyester fabric in the same conditions applied to the catalytic fabrics. During the first 40 min a slight adsorption was observed. Thereafter, absorbance increased with the increasing due to the evaporation of part of the water due to the heat generated by the UV lamp. The final absorbance of the solution was higher than the initial one, this discards degradation of Rh-B by UV irradiation. Adsorption was also minimal as explained. Fig. 2 shows, for different irradiation times in the presence of polyester-RGO + TiO2 fabric, the evolution of the absorption spectra of Rh-B solutions.
|
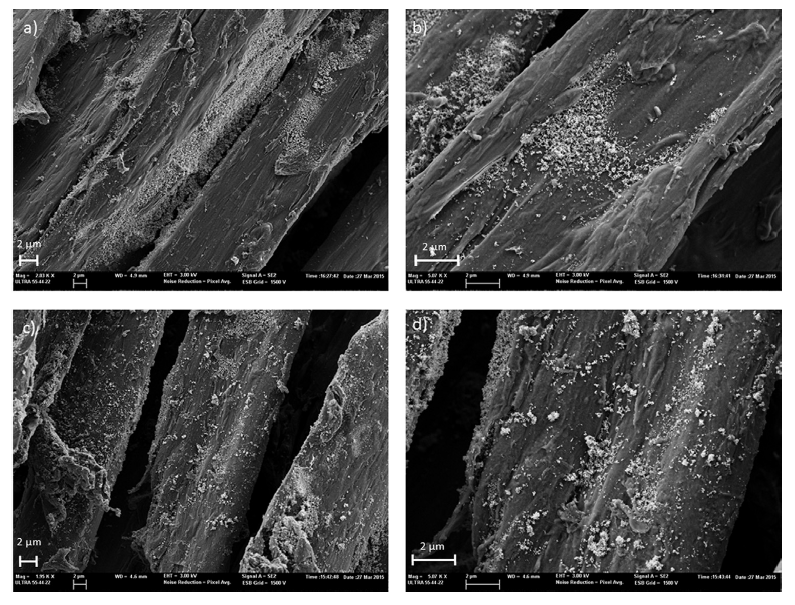 | Figure 3. FESEM micrographs of polyester-4G + TiO2 prior (a, b) and after performing the photocatalytic tests (c, d) |
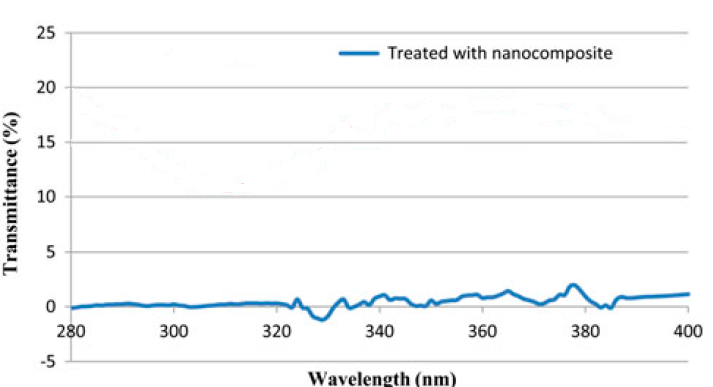 | Figure 4. UV transmittance spectra of treated polyester fabric with RGO/TiO2 nanocomposite |
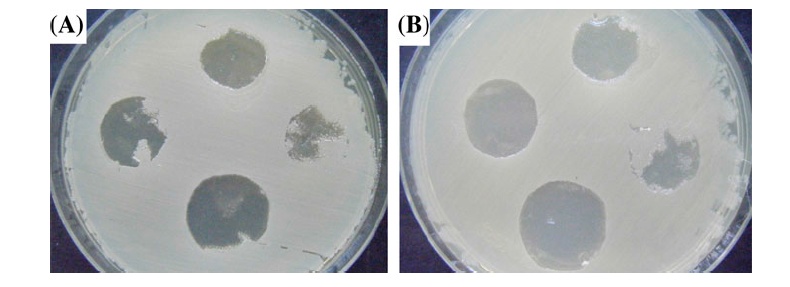
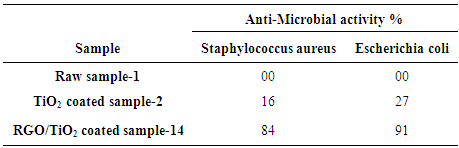
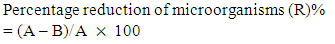 Where A and B are the number of micro-organisms colonies on untreated and treated fabrics, respectively. There was 3.4 × 105 colony forming units (cfu) of bacteria in the primary inoculum. Saline solution 8.5 g/L sodium chloride to 1000 ml distilled water was used as the neutralizing solution. Serial dilution of 10–10,000 was made for incubation on agar plate. Tryptic soy agar was applied as the agar.
Where A and B are the number of micro-organisms colonies on untreated and treated fabrics, respectively. There was 3.4 × 105 colony forming units (cfu) of bacteria in the primary inoculum. Saline solution 8.5 g/L sodium chloride to 1000 ml distilled water was used as the neutralizing solution. Serial dilution of 10–10,000 was made for incubation on agar plate. Tryptic soy agar was applied as the agar.
|
|
4. Conclusions
- As showed, a simple method based on polyester fabric coated with reduced graphene oxide/titanium dioxide nanocomposite was developed to produce multifunctional cellulose textiles. Coating graphene oxide/titanium dioxide nanocomposite on the polyester fabrics created functional characteristics including photocatalytic self-cleaning, antimicrobial, electrical conductivity, and UV blocking. Different numbers of RGO coatings were applied (1–4) to see the influence of this parameter on photocatalytic properties. FESEM showed the formation of the RGO/TiO2 coatings on the fabrics. No significant variations were observed between the fabrics with different number of RGO layers. It was difficult to characterize the surface TiO2 content due to the irregularity of the fabrics and the uneven distribution of the TiO2 nanoparticles.An increase in the photocatalytic degradation efficiency (η) of Rhodamine B was observed with the increasing number of RGO layers. This increase can be attributed to a higher light absorption, better conductivity, the decrease in the charge transfer resistance and the decrease of the time constant, which allows a better electron transfer between TiO2 nanoparticles and RGO-coated fabrics. It is expected that the graphene oxide/TiO2 nanocomposite might be used to produce high-performance fabrics and smart textiles.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
 . Applied power radiation: 300 W
. Applied power radiation: 300 W