-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2017; 7(1): 9-13
doi:10.5923/j.nn.20170701.03

Structural and Electronic Properties of SWGaPNT Drug Carrier
Bahjat B. Kadhim, Haider O. Muhsen
Department of Physics, College of Science, Al-Mustansiriyah University, Baghdad, Iraq
Correspondence to: Haider O. Muhsen, Department of Physics, College of Science, Al-Mustansiriyah University, Baghdad, Iraq.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The interaction of drug 5-fluorouracil with (4, 0) single wall Gallium-Phosphide nanotube (SWGaPNT) have been studied by using quantum mechanics. All of the calculations have been performed using a hybrid density functional method (DFT/B3LYP) and 6-31G∗ standard basis set. Quantum molecular descriptors and frontier orbital analysis in the drug nanotube systems were studied. (SWGaPNT) and it's applied as drug-delivery is also discussed. Results show that the SWGaPNT can act as a suitable drug delivery vehicle of 5-fluorouracil within biological systems.
Keywords: Density functional theory (DFT), SWGaPNT, Drug-delivery, 5-fluorouracil (5FU)
Cite this paper: Bahjat B. Kadhim, Haider O. Muhsen, Structural and Electronic Properties of SWGaPNT Drug Carrier, Nanoscience and Nanotechnology, Vol. 7 No. 1, 2017, pp. 9-13. doi: 10.5923/j.nn.20170701.03.
1. Introduction
- The emerging field of nanotechnology offers enormous potential to existing areas of biotechnology and medical technology innovations [1]. The major aim of nanomedicine is the design of material capable of delivery and targeting of pharmaceutical, therapeutic, and diagnostic agents [2]. Nanotechnology techniques are being used to fabricate and control nanobiomaterials structure and medical nanodevices investigating theproperties of matter at sizes below 100 nm [1]. Nanomedicine is the branch of nanotechnology and nanoscience that would allow the ability to cure disease from inside the body and at the cellular or molecular level; it is one of the most promising fields within the potential new technological advances in medicine [3]. There have been recent advances in the use of nanotechnology in detection, disease diagnosis, imaging, monitoring, therapeutics and drug-delivery systems in modern medicine [4]. Ever since the discovery of carbon nanotubes (CNTs) by Iijima [1] considerable efforts have been performed to investigate their electronic and structural properties in which the dependencies on tubular diameter and chirality have been recognized [5]. The physical and chemical properties of nanostructures such as nanotubes, nanowires, and Nanoclusters can be tuned by Varying their sizes, geometries, and chemical compositions. The knowledge of these variations plays important role while downscaling the size of Nanodevices [6].Many investigations centered on non-carbon-based nanotubes, which exhibit electronic properties different from the properties of their carbon relatives. Among them, from the groups three and five of the periodic table, which are the neighbors of C, boron nitride (BN), aluminum nitride (AlN), gallium nitride (GaN), indium nitride (InN), boron phosphide (BP), aluminum phosphide (AlP), gallium phosphide (GaP), and indium phosphide (InP) [7]. It’s had stabilized tubular structures [8]. These nanotubes are inorganic analogs of CNTs and display physical properties suited to a broad variety of applications, and these nanotubes behave as semiconductors in every case. Also, the nanotubes are being considered as more appropriate materials than the CNTs [7]. Gallium phosphide (GaP) is a popular semiconductor with a wide band gap of 2.26 eV, GaP nanotubes are polycrystalline with zinc blende structure [9].5-fluorouracil molecule (5-FU) is a fluorinated pyrimidine analogue chemotherapeutic agent using as solid cancer treatment like esophagus, stomach, intestines, carcinoma [10]. It has been used as an anti-cancer drug for 40 years. The structure of fluorouracil is observed in (Figure. 1). It is anti-metabolite drug and acts in several ways, but principally as synthesis inhibitor. These days there are ways to deliver a drug in the body without side effects [2]. In this study, a theoretical study, we reported types of drug delivery system such as GaP nanotube. The structural stabilities of GaP nanotubes (GaPNTs) are examined by performing density functional theory (DFT) calculations on the representative (4, 0) zigzag models (Figure 1). The atomic geometries are allowed to relax by optimization.
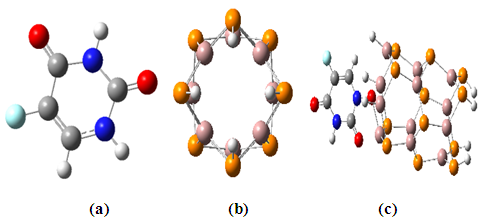 | Figure 1. The structures of optimized molecular by B3LYP/6-31G* method (a) Fluorouracil, (b) Nanotube (4, 0), and (c) Complex |
2. Computational Detail
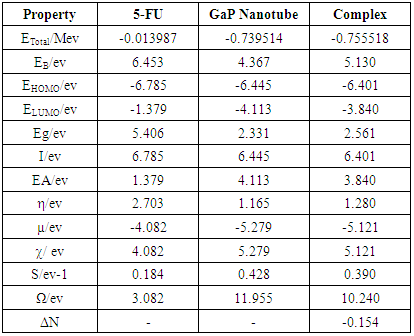
- All of the calculations were carried out using a personal computer has name Lenovo G50 which has processor Intel core (TM) i7-5560u CPU 2.4 GHZ 2.4 GHZ with 8-GB RAM at 37°C. The representative zigzag model of the single-walled GaPNT is considered within this work. The diameter and length of (4, 0) SWGaPNT are about 6.95 and 9.50 Å, respectively. SWGaPNTs consisting of 32 atoms (12 Gallium atoms, 12 Phosphorus atoms, and 8 hydrogen atoms). Hydrogen atoms are used to saturate the Ga and P atoms to avoid dangling effects at the two ends by keeping three covalent bonds for each Ga and P atoms of nanotubes [8] (Figure 1b). A nanotube is formed using a nanotube modeler package [11]. The selected drug (5-fluorouracil) were made using Gauss View. The structures (complexes of the single-walled nanotube with fluorouracil) are individually optimized employing DFT/ B3LYP exchange-functional and 6-31G∗ standard basis set by Gauss View and Gaussian 09 program package [12] (Figure 1).The bond length (Å), total energy, band gap and HOMO/LUMO were investigated in the composites. The Binding energy (EB) of SWGaPNT and complexes are obtained from equation (1) [13].
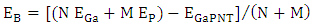 | (1) |
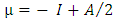 | (2) |
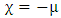 | (3) |
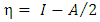 | (4) |
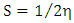 | (5) |
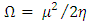 | (6) |
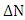 method:
method: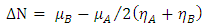 | (7) |
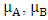 and
and  are the chemical potential and the chemical hardness of the systems A and B. A positive value of
are the chemical potential and the chemical hardness of the systems A and B. A positive value of  indicates that charge flows from nanotube to the drug and the drug act as an electron acceptor, while a negative value of
indicates that charge flows from nanotube to the drug and the drug act as an electron acceptor, while a negative value of  indicates that charge flows from the drug to nanotube and the drug acts as an electron donor [14].
indicates that charge flows from the drug to nanotube and the drug acts as an electron donor [14].3. Results and Discussion
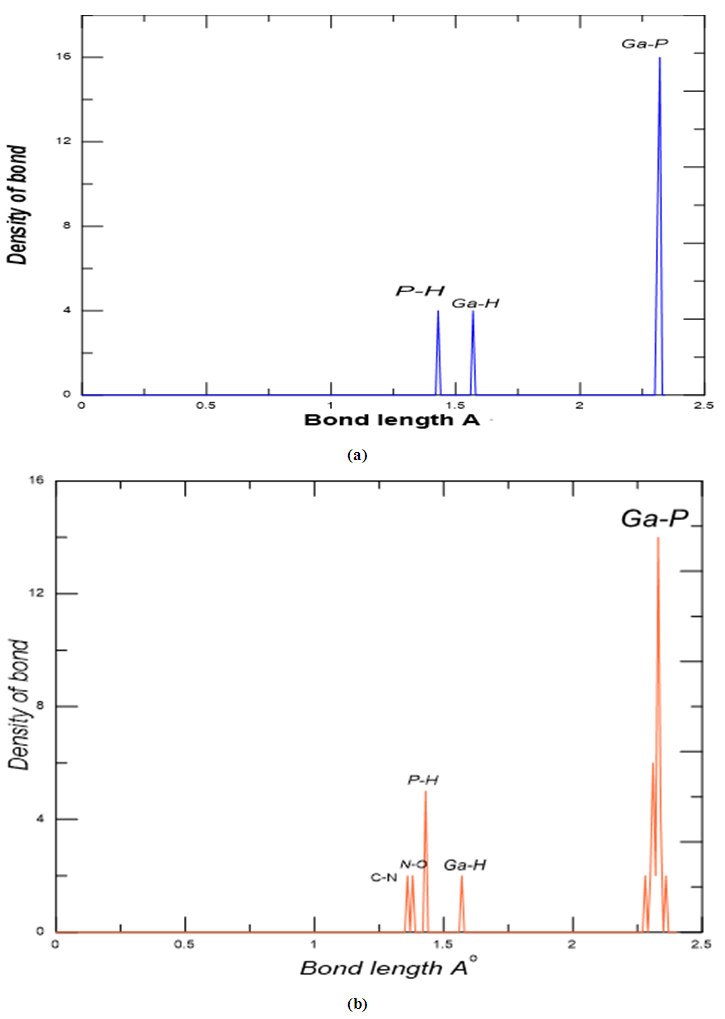
- First of all, we discussed the structural and electronic properties of the zigzag (4, 0) SWGaP nanotube and SWGaPNT coupled to 5-fluorouracil (5-FU) molecule (see Figure 1). The optimized structure parameters of GaP nanotube and composite calculated by DFT-B3LYP levels with the 6-31G* basis.From Figure (2.a) the optimized structure of the pristine SWGaPNT has the Ga-P bond length 2.30 Å, the presented results in this work are in agreement approximately with previous studies provided by Anurag Srivastava et al [14]. It can be noticed that the bond length at about 1.42 Å and 1.56 Å for (P-H) and (Ga-H) bonds respectively, the presented results in this work are in agreement with previous studies provided by Maryam Mirzaei and Mahmoud Mirzae [5]. As seen in Figure (2b) that the averaged values for Ga–P bond lengths are 2.31 Å which is slightly longer than those in the pristine model for nanotube. The values for Ga–H and P–H bonds do not show notable changes. With an additional bond density equal to 1.36 Å and 1.39 Å for (C-N) and (N-O) bonds. While the tetrahedral angle of Ga-O-N is 108.28° which is represent couple angle of the drug with the GaP -nanotube.
|
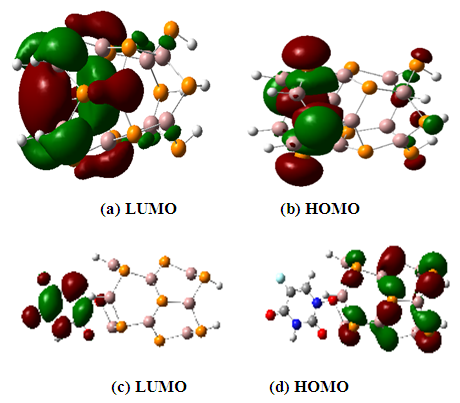 | Figure 3. HOMO and LUMO level of. (a) LUMO of GaPNT. (b) HOMO of GaPNT and (c) LUMO of Complex, and (d) HOMO of complex |
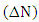 method, is given in table 1. A positive value of
method, is given in table 1. A positive value of 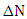 indicates that the (5-FU) act as an electron acceptor, while a negative value of
indicates that the (5-FU) act as an electron acceptor, while a negative value of 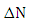 indicates that the (5-FU) act as an electron donor. From the results
indicates that the (5-FU) act as an electron donor. From the results 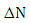 value is negative, indicating that (4, 0) SWGaPNT act as electron acceptors.
value is negative, indicating that (4, 0) SWGaPNT act as electron acceptors.4. Conclusions
- Using density functional theory, the effects of the coupled of (5-FU) with single wall Gallium Phosphide nanotube (SWGaPNT) have been studied in details. The total energies for complex cause decreasing in energy and more stable structure. A small energy gap means small excitation energies of manifold of the excited states. The capability of a nanotube to accept precisely one electron from a donor is measured by its electron affinity (EA). The strength of an accepter molecule is measured by its electron affinity (EA) which the energy released when adding one electron to LUMO. An accepter must have a high EA. FMO and structural analyses show that the low energy level of LUMO. Decrease in global hardness, energy gap, and ionization potential and also, increase in electron affinity and electrophilicity of the complexes shows a charge transfer from the (5-FU) molecule to the nanotube model.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML