| [1] | Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005; 26: 3995–4021. |
| [2] | Xie J, Xu CJ, Xu ZC, Hou YL, Young KL, Wang SX, Linking hydrophilic macromolecules to monodisperse magnetite (Fe3O4) nanoparticles viatrichloro-s-triazine. Chem Mater 2006; 18: 5401–3. |
| [3] | Arruebo M, Fernandez-Pacheco R, Ibarra MR, Santamaria J. Magnetic nanoparticlesfor drug delivery. Nano Today 2007; 2: 22–32. |
| [4] | Mornet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues Magnetic nanoparticle design for medical applications. In: Meeting of the European-Materials- Research-Society. Strasbourg, France: Pergamon-Elsevier Science Ltd.; 2005. p. 237–47. |
| [5] | Reshmi G, Kumar PM, Malathi M. Preparation, characterization and dielectric studies on carbonyl iron/cellulose acetate hydrogen phthalate core/shell nanoparticles for drug delivery applications. Int J Pharm 2009; 365: 131–5. |
| [6] | Arias JL, Lopez-Viota M, Ruiz MA, Lopez-Viota J, Delgado AV. Development of carbonyl iron/ethylcellulose core/shell nanoparticles for biomedical applications. Int J Pharm 2007; 339: 237–45. |
| [7] | Wei-Chen Huang, Tai-Jung Lee, Chi-Sheng Hsiao, San-Yuan Chen and Dean-Mo Liu. Characterization and drug release behavior of chip-like amphiphilic chitosan–silica hybrid hydrogel for electrically modulated release of ethosuximide. J.Mater. Chem., 2011,21, 16077-16085 |
| [8] | Fernandez-Pacheco R, Marquina C, Valdivia JG, Gutierrez M, Romero MS, Cornudella R. Magnetic nanoparticles for local drug delivery using magnetic implants. In: 6th international conference on the scientific and clinical applications of magnetic carriers. Elsevier Science B.V.; 2006. p. 318–22. |
| [9] | Cregg PJ, Murphy K, Mardinoglu A. Calculation of nanoparticle capture efficiency in magnetic drug targeting. J Magn Magn Mater 2008; 320: 3272–5. |
| [10] | Wang D, Li J, Li H, Tang F. Preparation and drug releasing property of magnetic chitosan-5-fluorouracil nano-particles. Trans Nonferrous Metals Soc China 2009; 19:1232–6. |
| [11] | Faraji A, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem 2009; 17:2950–62. |
| [12] | Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol 2009; 86:215–23. |
| [13] | Bhattarai SR, Badahur KCR, Aryal S, Khil MS, Kim HY. N-acylated chitosan stabilized iron oxide nanoparticles as a novel nano-matrix and ceramic modification. Carbohydr Polym 2007; 69: 467–77. |
| [14] | Chertok B, Moffat BA, David AE, Yu FQ, Bergemann C, Ross BD, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRImonitored magnetic targeting of brain tumors. Biomaterials 2008; 29:487–96. |
| [15] | Kumar A, Jena P, Behera S, Lockey R, Mohapatra S, Mohapatra S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine 2009:1–6. |
| [16] | M. Asplund, T. Nyberg and O. Inganas, “Electroactive Polymers for Neutral Interfaces,” Polymer Chemistry, Vol. 1, No. 9, 2010, pp. 1374-1391. doi:10.1039/c0py00077a. |
| [17] | T. Skotheim and J. R. Reynolds, “Handbook of Conduct-ing Polymers: Conjugated Polymers Processing and Ap-plications 3rd Edition,” Taylor and Francis, New York, 2007. |
| [18] | S. H. Gehrke and P. I. Lee, “Hydrogels for Drug Delivery Systems,” In: P. Tyle, Ed., Specialized Drug Delivery Systems, Marcel Dekker, New York, 1990, pp. 333-392. |
| [19] | P. Gupta, K. Vermani and S. Garg, “Hydrogels: From Controlled Release to pH Responsive Drug Delivery,” Drug Discovery Today, Vol. 7, No. 10, 2002, pp. 569-579. |
| [20] | N. Wisniewski, F. Moussy and W. M. Reichert, “Charac-terization of Implantable Biosensors Membrane Bio-fuling,” Fresenius’ Journal of Analytical Chemistry, Vol. 366, 2000, pp. 611-621. |
| [21] | O. M. Schuvailo, O. O. Soldatkin, A. Lefebvre, R. Cespuglio and A. P. Soldatkin, “Highly Selective Micro-biosensors for in Vivo Measurement of Glucose, Lactate and Glutamate,” Analytical Chimica Acta, Vol. 573-574, 2006, pp. 110-116. doi:10.1016/j.aca.2006.03.034 |
| [22] | P. M. George, A. W. Lyckman, D. A. LaVan, A. Hegde, Y. Leung, R. Avasare, C. Testa, P. M. Alexander, R. Langer and M. Sur, “Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics,” Biomaterials, Vol. 26, No. 17, 2005, pp. 3511-319. |
| [23] | X. Cui, J. Wiler, M. Dzaman, R. A. Altschuler and D. C. Martin, “In Vivo Studies of Polypyrrole/Peptide Coated Neural Probes,” Biomaterials, Vol. 24, No. 5, 2003, pp. 777-787. doi:10.1016/S0142-9612(02)00415-5. |
| [24] | B. Zinger and L. L. Miller, “Timed Release of Chemicals from Polypyrrole Films,” Journal of the American Chemical Society, Vol. 106, No. 22, 1984, pp. 6861-6863. doi:10.1021/ja00334a076. |
| [25] | N. K. Lau and L. L. Miller, “Electrochemical Behavior of a Dopamine Polymer. Dopamine Release as a Primi-tive Analog of a Synapse,” Journal of the American Chemical Society, Vol. 105, No. 16, 1983, pp. 5271-5277. doi:10.1021/ja00354a016. |
| [26] | S. Murdan, “Electro-Responsive Drug Delivery from Hydrogels,” Journal of Controlled Release, Vol. 91, 1994, pp. 3201-3204. |
| [27] | A. Guiseppi-Elie and N. F. Sheppard Jr., “Conferrig Bio-specificity to Electroconductive Polymer-Based Biosen-sors Devices,” ACS Northeast Regional Meeting (NERM), Rochester, October 1995, pp. 22-25. |
| [28] | C. J. Small, C. O. Too and G. G. Wallace, “Responsive Conducting Polymer-Hydrogel Composites,” Polymer Gels and Network, Vol. 5, No. 3, 1997, pp. 251-265. doi:10.1016/S0966-7822(96)00044-5. |
| [29] | B. Zinger and L. L. Miller, “Timed Release of Chemicals from Polypyrrole Films,” Journal of the American Chemical Society, Vol. 106, No. 22, 1984, pp. 6861-6863. doi:10.1021/ja00334a076. |
| [30] | A.N. K. Lau and L. L. Miller, “Electrochemical Behavior of a Dopamine Polymer. Dopamine Release as a Primi-tive Analog of a Synapse,” Journal of the American Chemical Society, Vol. 105, No. 16, 1983, pp. 5271-5277. doi:10.1021/ja00354a016. |
| [31] | X. Cui, V. A. Lee, Y. Raphael, J. A. Wiler, J. F. Hetke, D. J. Anderson and D. C. Martin, “Surface Modification of Neutral Recording Electrodes with Conducting Polymer/ Biomolecule Blends,” Journal of Biomedical Materials Research, Vol. 56, No. 2, 2001, pp. 261-272. |
| [32] | J. Liu, J. Shi, L. Jiang, F. Zhang, L. Wang, & S. Yamamoto, Segmented magnetic nanofibers for single cell manipulation, Appl. Surf. Sci., Vol. 258, 7530–7535, (2012). |
| [33] | Issam A. Latif, Hilal M. Abdullah, Maida Hameed Saleem, Electrical and Swelling Study of Different Prepared Hydrogel. American Journal of Polymer Science 2016, 6(2): 50-57. |
| [34] | J. Stejskal & R. G. Gilbert, Polyaniline. Preparation of a conducting Polymer (IUPAC Technical Report), Pure Appl. Chem., Vol. 74, No. 5, 857–867, (2002). |
| [35] | Seyfullah Madakbas, Kadir Esmer, Ersin Kayahan, & Mehmet Yumak “Synthesis and Dielectric Properties of Poly(aniline)/Na-bentonite Nanocomposite”, Science and Engineering of Composite Materials 17, 145-153(2010). |
| [36] | U. Edlund, and A. C. Albertsson, “Degradable Polymer Microspheres for Controlled Drug Delivery,” Advances in Polymer Science, Vol. 157, 67-112(2002). |
| [37] | D. Sahoo, S. Sahoo, J. Das, T. K. Dangar and P. L. Na- yak, “Antibacterial Activity of Chitosan Crosslinked with Aldehydes and Blended with Cloisite 30B,” NanoTrends, Vol. 10, 1-9(2011). |
| [38] | Sajjad Sedaghat, Synthesis and characterization of new bio compatible copolymer: chitosan -graft-polyaniline, International Nano Letters, 4: 2, 1-6 (2014). |
| [39] | Karina Donadel, Marcos D.V. Felisberto, Valfredo T. Fávere, Mauricio Rigoni, Nelson Jhoe Batistela, Mauro C.M. Laranjeira, Synthesis and characterization of the iron oxide magnetic particles coated with chitosan biopolymer, Materials Science and Engineering C 28, 509–514(2008). |
| [40] | Liang Guo, Guang Liu , Ruo-Yu Hong , and Hong-Zhong Li, Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres, Mar. Drugs, 8, 2212-2222 (2010). |
| [41] | A. R. Subrahmanyam, V. Geetha, Atul kumar, A. Alakanandana, J. Siva Kumar, Mechanical and Electrical Conductivity Studies of PANI-PVA and PANI-PEO Blends, International Journal of Material Science, Vol.2, Iss.1, PP.27-30(2012). |
| [42] | Liu L, Xiao L, Zhu HY, & Shi XW, Preparation of magnetic and fluorescent bifunctional chitosan nanoparticles for optical determination of copper ion. Microchim Acta, 178(3): 413–419 (2012). |
| [43] | Tsangaris GM, Psarras GC, & Kouloumbi N "Polyurethane latex/water dispersible boehmite alumina nanocomposites: Thermal, mechanical and dielectrical properties", Journal of Materials Science, vol. 33, no.8, 2027-2037, (1998). |
| [44] | K.G. Gatos, J.G. Martinez Alcazar, G.C. Psarras, R. Thomann, & J. Karger-Kocsis, Polyurethane latex/water dispersible boehmite alumina nanocomposites: thermal mechanical and dielectrical properties, Composites Science and Technology 67 (2) 157–167(2007). |
| [45] | Psarras GC, Gatos KG, Karahaliou PK, Georga SN, Krontiras CA, & Karger-Kocsis J "Relaxation phenomena in rubber/ layered silicate nanocomposites", Springer, Express Polymer Letters, vol. 1, 837-845(2007). |
| [46] | Kalini A, Gatos KG, Karahaliou PK, Georga S N, Krontiras CA, & Psarras GC "Probing the dielectric response of polyurethane/alumina nanocomposites", Journal of Polymer Science Part B: Polymer Physics, vol. 48, 2346-2354 (2010). |
| [47] | Li YC, Li RKY, & Tjong SC "Frequency and temperature dependences of dielectric dispersion and electrical properties of polyvinylidene fluoride/expanded graphite composites "javascript; Springer, Journal of Nanomaterials, no vol., and pp. given, (2010). |
| [48] | Dang Z, Wang L, Yin Y, Zhang Q, Lei Q "Giant dielectric permittivities in functionalized carbonnanotube/ electroactive - polymer nanocomposites", Wiley, Advanced Materials, vol. 19, pp.852-857, (2007). |
| [49] | S. Devikala, P. Kamaraj and M. Arthanareeswari, Conductivity and Dielectric Studies of PMMA Composites, Chem Sci Trans., 2(S1), S129-S134 (2013). |
| [50] | Bajpai, A.K., Shukla, S.K., Bhanu, S., & Kankane, S., “Responsive polymers in controlled drug delivery”. Prog. Polym. Sci., 33, 1088–1118(2008). |
| [51] | Li Y., Neoh K.G., Kang E.T., Controlled release of heparin from polypyrrole-poly(vinyl alcohol) assembly by electrical stimulation. J. Biomed. Mater. Res., 73A, 171–181(2005). |
| [52] | M. Filippousi, T. Altantzis, G. Stefanou, M. Betsiou, D. N. Bikiaris, M. Angelakeris, E. Pavlidou, D. Zamboulis and G. Van Tendeloo, “Polyhedral iron oxide core–shell nanoparticles in a biodegradable polymeric matrix: preparation, characterization and application in magnetic particle hyperthermia and drug delivery”, RSC Adv., 3, 24367–24377. |



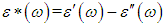
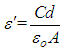
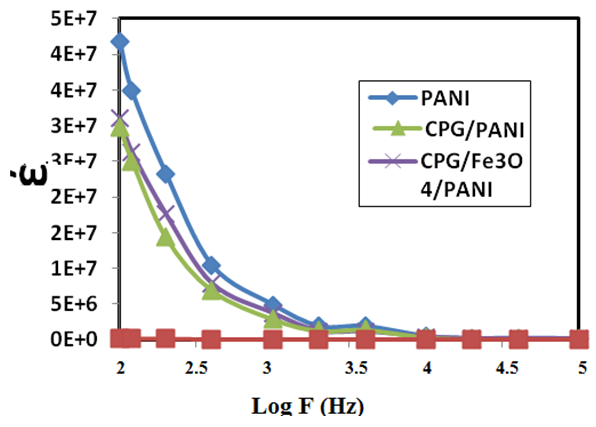
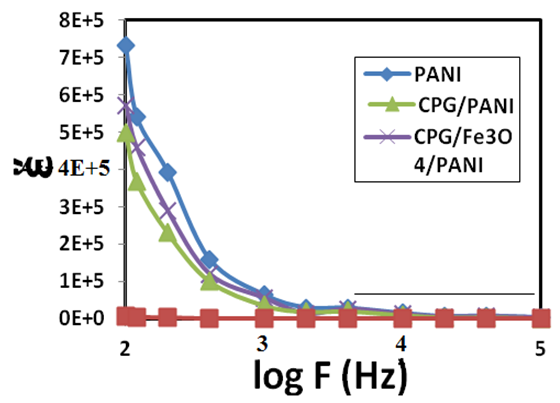
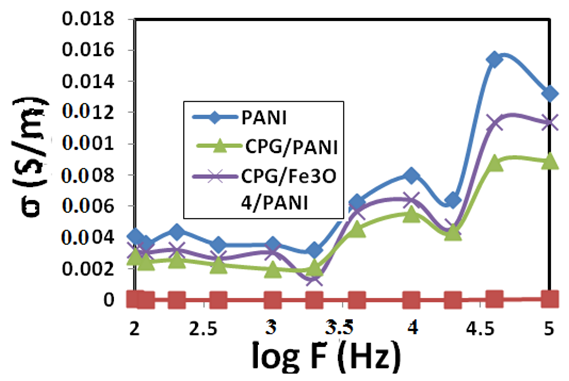
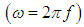 ω and f were mean the angular frequency and the applied frequency respectively. While for the real and imaginary permittivity ε'(ω), ε" (ω) and tanϬ was tangent delta,
ω and f were mean the angular frequency and the applied frequency respectively. While for the real and imaginary permittivity ε'(ω), ε" (ω) and tanϬ was tangent delta,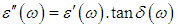
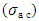 calculated with the equation 4 [35].
calculated with the equation 4 [35].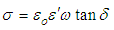
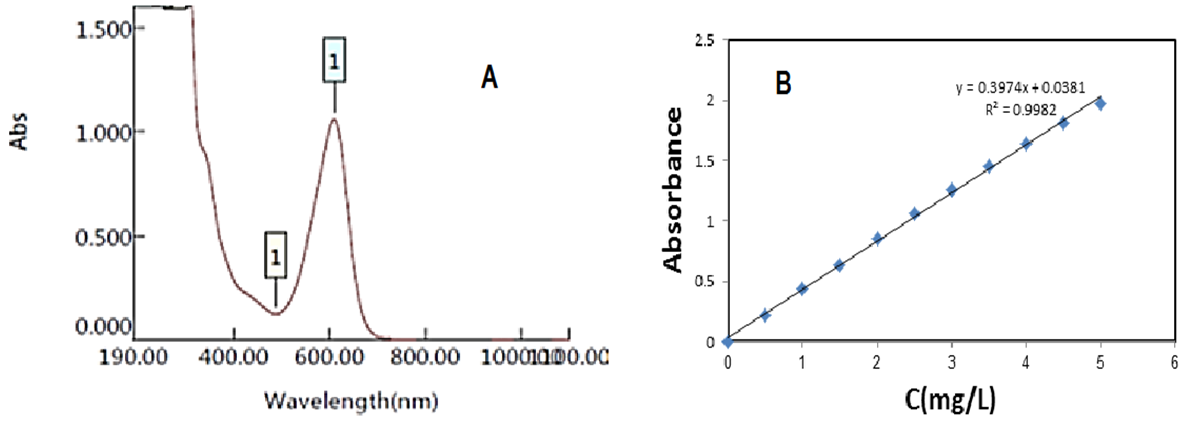
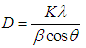
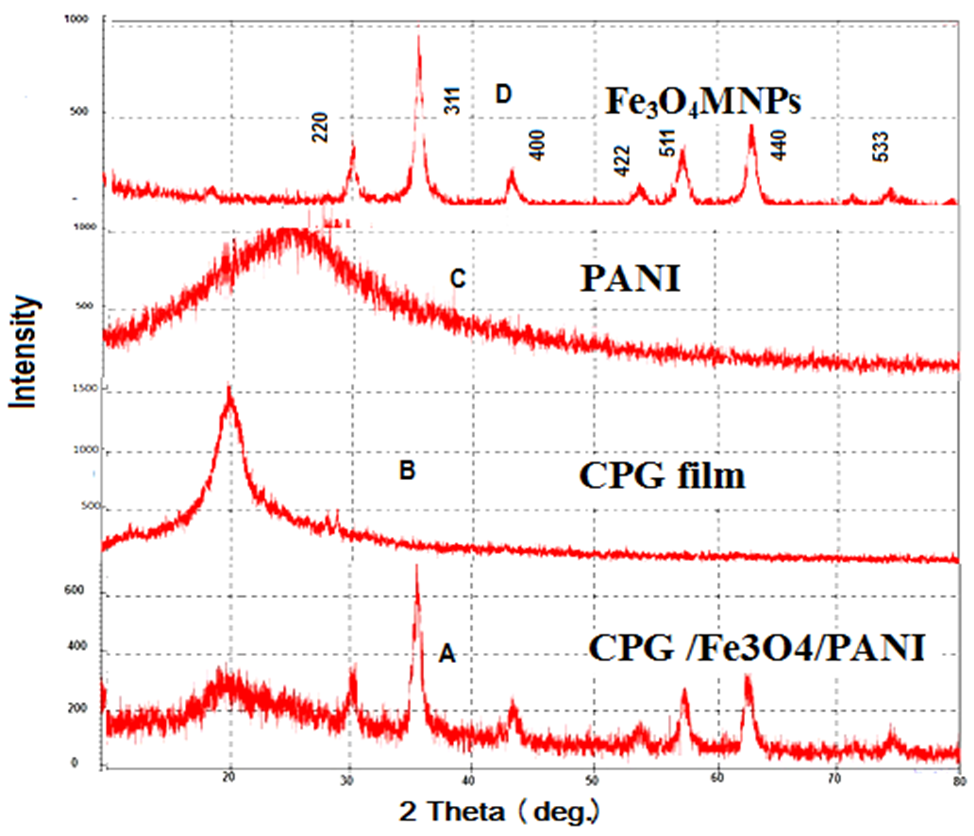
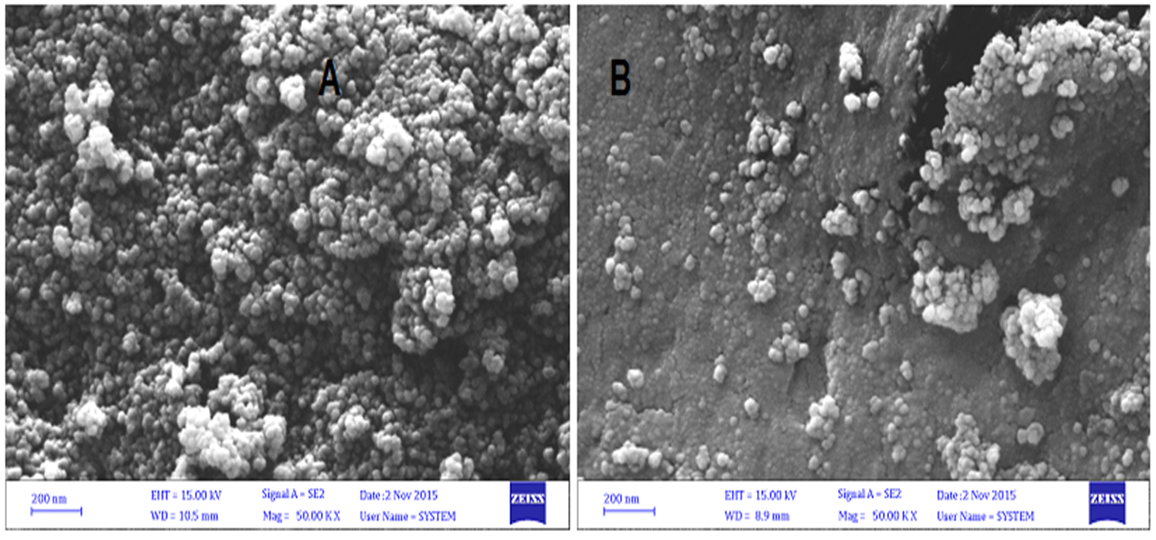
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML