-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(3): 43-47
doi:10.5923/j.nn.20160603.02

Novel Polyamide Nanocomposite with Montmorillonite Clay and Gold Nanoparticle Nanofillers
Ayesha Kausar
Nanosciences Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this attempt, a novel polyamide was prepared using 2-aminoethanethiol hydrochloride and 2,6-pyridinedicarbonyl dichloride. The gold nanoparticle (GNP) was prepared using simple salt precipitation method. Later the polyamide/gold nanoparticle (PA/GNP) and the nanocomposite incorporating gold and montmorillonite (PA/MMT/GNP) were fabricated. The molecular weight, morphology, thermal stability, and non-flammability properties were studied using relevant techniques. The nanocomposite was found to have uneven porous fractured surface morphology according to scanning electron microscopy (SEM). The thermogravimetric analyses indicated that the aromatic polyamide was stable up to 399°C (Tmax), whereas the nanocomposite had higher thermal stability of 470-510°C. Whereas the PA/GNP 0.1-0.5 series depicted lower Tmax of 411-435°C. Moreover, the LOI measurement indicated higher non-flammability of 40-63% (PA/MMT/GNP 0.1-0.5) relative to neat polyamide (24%) and PA/GNP (29-35 %).
Keywords: Polyamide, Nanocomposite, Gold nanoparticle, Montmorillonite, LOI measurement
Cite this paper: Ayesha Kausar, Novel Polyamide Nanocomposite with Montmorillonite Clay and Gold Nanoparticle Nanofillers, Nanoscience and Nanotechnology, Vol. 6 No. 3, 2016, pp. 43-47. doi: 10.5923/j.nn.20160603.02.
Article Outline
1. Introduction
- After polyester and cotton, polyamide (PA) is the third largest textile fiber utilized in the world. The research for novel materials with enhanced features and potential is an extent in constant development [1, 2]. Polyamides are amongst the most common polymers in our daily life: The thermal and mechanical features make their employment spread in numerous fields, ranging from textile and fabric industries to technological applications. The amide group related to their backbone could act as a ligand for metals and thus they are provided for metal nanoparticle stabilization. The polyamide metal nanoparticle composites visualize their synthesis by means of top-down approaches, while bottom-up approaches are not considered, though in standard simpler and faster [3]. For the synthesis of metal nanoparticles-polymer composites, three general methods have been reported. The first method comprises the in-situ synthesis of nanoparticles in the polymer matrix. This is frequently attained by decreasing a metal salt already existing in the matrix or by evaporating the metal at the heated surface of the matrix. The second route comprises the polymerization of the matrix around the nanoparticles. This approach, moreover, yields polydisperse polymer matrixes. In-situ synthesis of nanoparticles is also capable to produce polydisperse and uncontrolled nanoparticle sizes. Furthermore, undesired species in the matrices are synthesized by both these techniques, either from the polymerization or the reduction steps. The third route employed for synthesis of metal nanoparticle-polymer composites was currently reported from our laboratories. This is possibly associated to the insolubility of polyamide in several of the most common solvents, which inhibits to have uniform solutions, required for exploitation of wet chemical synthesis. The strengthening with filler is principally significant for polymers from renewable resources, since most of them have the disadvantages of lower softening temperatures and lower modulus [4, 5]. Additionally, the hydrophilic performance of most natural polymers presents an important advantage, since it offers a compatible interface with the nanoclay [6]. Special consideration has been paid to montmorillonite (MMT) minerals in the field of nanocomposites due to their small particle size, tremendously large surface areas, and intercalation features [7-9]. MMT is comprised of silicate layers that are 1 nm thick in planar structure and 200-300 nm in the lateral dimension. The distinctive chemical structures of MMT frequently comprise of two merged silica tetrahedral sheets sandwiching an edge-shared octahedral sheet of either magnesium or aluminum hydroxide [10, 11]. At low level of nanofiller integration (less than 5 wt.%), the strengthening efficacy of nanocomposite can match that of conservative composites with 40-50 wt.% of loading with conventional fillers. This enhancement is because of the dispersal of nanoscale fillers into the matrix, which results in a high surface area with high interactions between nanofillers and the polymer matrix [12]. Amongst all the thermoplastic polymers utilized as a support, a little consideration has been devoted to polyamide. This polymer, categorized in the engineering semi-crystalline thermoplastic family, comprises outstanding thermal, mechanical and chemical resistance features [13, 14]. Additionally, this type of polymer can easily form spherical nanostructures called spherulites by crystallization process from solution [15-17]. Therefore, polyamide has been measured as a best matrix for the loading of gold nanoparticle and silicate filler materials. In the present work, a polyamide was formed by the reaction of 2-aminoethanethiol hydrochloride and 2,6-pyridinedicarbonyl dichloride. The matrix was then reinforced with the two types of nanofiller i.e. montmorillonite (MMT) and gold nanoparticle (GNP). Afterwards the materials were characterized for morphology, thermal and non-flammability properties.
2. Experimental
2.1. Materials
- Montmorillonite K10 powder, 2-aminoethanethiol hydrochloride (98%), 2,6-pyridine-dicarbonyl dichloride (97%), poly(ethylene glycol) (Mn ~400), HAuCl4 (10 nm particle size), 2,2'-azoisobutyronitrile (AlBN, ≥98%), 4-(4-aminophenylsulfonyl)benzenamine (APSBA, 97%), ammonium thiocyanate (98%), dimethyl formamide (DMF, 99%), and sodium carbonate were obtained from Aldrich.
2.2. Characterization Techniques
- FTIR Spectrometer FTSW 300 MX (BIO-RAD) was used to scan the spectra of samples in the range of 400-4000 cm-1. Gel permeation chromatography (GPC) was used to evaluate the weight-average molecular weight (Mw) of the polymer using DMF as an eluent. The micrographs were obtained by Scanning Electron Microscope S-4700 (Japan Hitachi Co. Ltd.). The thermal stability was determined by NETZSCH thermo gravimetric analyzer (TGA), model no. TG 209 F3 (California, USA), using 5 mg of sample in Al2O3 crucible. The temperature range was taken from 0 to 800C at heating rate of 10°C/min under nitrogen atmosphere. The LOI measurements were performed on a Stanton Redcroft FTA flammability unit provided with an Oxygen Analyzer.
2.3. Synthesis of Gold Nanoparticle (GNP)
- 0.05 g Na2CO3 was dissolved in 200 mL deionized water. The solution was added to 30 mL HAuCl4 aqueous solution (3×10-3 M) and stirred for 0.5 h. Then 0.05 g PEG (10 wt.%) emulsion and 1 mL formaldehyde (35 wt. %) were added to the above mixture and stirred for 1 h. The resulting gold nanoparticles were isolated by centrifugation. The nanoparticles were re-dispersed in ethanol and deionized water and separated out by centrifugation [18].
2.4. Formation of Polyamide
- 2-Aminoethanethiol hydrochloride (0.01 mol) and 50 mL DMF were charged in 200 mL round bottom flask equipped with nitrogen. The mixture was placed in an ice bath. Anhydrous dichloromethane (50 mL) was added via syringe followed by triethylamine (5 mL). The mixture was stirred for 2 h. 2,6-Pyridinedicarbonyl dichloride (0.01 mol) was dissolved in 10 mL DMF and added drop wise to the reaction flask (Fig. 1). The mixture was precipitated in water and filtered to obtain the polyamide [19]. FTIR (KBr, cm-1): 3339 (N–H stretch), 1590 (N–H bend), 3015 (aromatic C–H stretch), 1680 (amide C=O stretch), 1414 (C–N stretch). Mw = 18,000 g/mol (GPC).
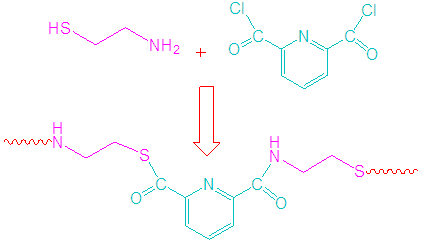 | Figure 1. Formation of polyamide |
2.5. Synthesis of Polyamide/Gold Nanoparticle Nanocomposite (PA/GNP)
- 1g polyamide was dispersed in 20 mL DMF. To the clear solution, 0.1 g gold nanoparticle was added. The mixture was stirred for 24 h to ensure the coordination between the organic and inorganic materials. Finally, the PA/GNP hybrid material was filtered and washed with distilled water. The product was dried at 100 °C for 6 h to remove excess water [20].
2.6. Fabrication of Polyamide/Montmorillonite/Gold Nanoparticle Nanocomposite (PA/MMT/GNP)
- 1g polyamide was dispersed in 20 mL DMF. To the clear solution, 0.1 g gold nanoparticle was added. Then the desired amount of MMT (0.1-0.5%) was added to the mixture. The reaction mixture was stirred for 24 h to ensure the coordination between organic and inorganic materials. Finally, the PA/MMT/GNP hybrid material was filtered and washed with distilled water (Fig. 2). The product was dried at 100°C for 6h to remove excess of water.
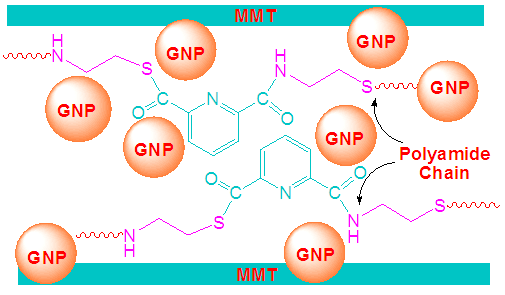 | Figure 2. Schematic of PA/MMT/GNP nanocomposite |
3. Results and Discussion
3.1. Morphological Study
- Field emission scanning electron microscopic (FE-SEM) study was performed on the samples including PA/MMT/GNP 0.1, PA/MMT/GNP 0.3, and PA/MMT/GNP 0.5. Fig. 3A depicts the morphology of 0.1 wt.% nanofiller loaded nanocomposite with somewhat unbalanced surface. The porous sheet-like morphology was observed for the fractured surface of nanocomposite. In the case of PA/MMT/GNP 0.3 nanocomposite, the nano-pores were more apparent on the fractured surface of the nanocomposite (Fig. 3B). However the nanocomposite surface was still uneven in texture. The morphology of PA/MMT/GNP 0.5 nanocomposite was somewhat different, because the nano-pores were less visible due to the appearance of small flakes on the fractured surface (Fig. 3C). In other words, the nanocomposite formed well-flaked off morphology. Moreover the nanofiller aggregates were not observed in the micrographs showing fine dispersion of gold and clay nanoparticle. This kind of morphology is often found for the polymer/clay nanocomposite presented in literature [21, 22]. SEM analysis showed the development of flake like morphology due to the inclusion of clay. The morphology can be further explored using high resolution transmission electron microscopy.
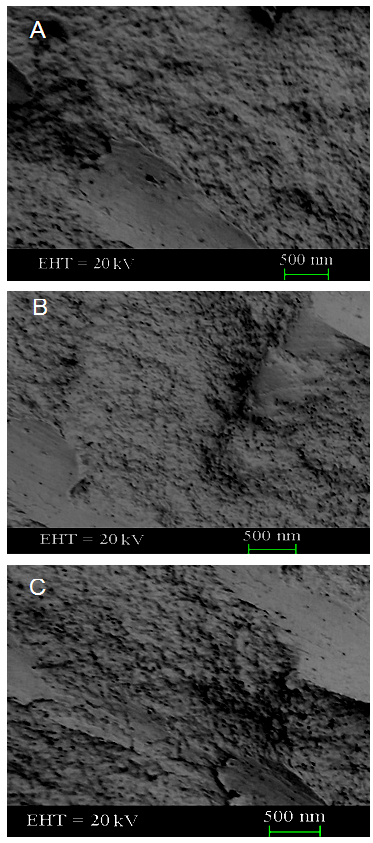 | Figure 3. FESEM images of (A) PA/MMT/GNP 0.1; (B) PA/MMT/GNP 0.3; and (C) PA/MMT/GNP 0.5 |
3.2. Thermal Stability
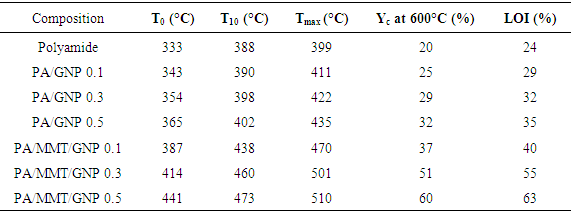
- Table 1 shows the thermal analysis data of neat polyamide and nanocomposite. The data include the onset degradation temperature (T0), the temperature at which 10% weight loss occurs (T10), maximum weight loss temperature, and char yield at 600°C. The T0 of polyamide was 333°C, T10 was 388°C, and Tmax was around 399°C (Fig. 4). For PA/MMT/GNP 0.1 nanocomposite, the onset temperature of degradation was 387°C while T10 was 438°C. The maximum degradation temperature for PA/MMT/GNP 0.1 was found as 470°C. There was an increase in the thermal stability of nanocomposite with nanofiller loading. The T0 of nanocomposite was 414°C, T10 was 460°C, and Tmax was around 501°C. The thermal stability of 0.5 wt.% nanocomposite was found higher than that of neat polyamide and other nanocomposite. Therefore further higher values for T0, T10 and Tmax were observed.The T0 was observed as 441°C, T10 was 473°C, and Tmax was around 510°C. While the PA/GNP 0.1-0.5 series depicted lower values in thermal properties as T0 = 343-365°C, T10 = 390-402°C, and Tmax = 411-435°C, compared with the MMT-GNP reinforced series. The results indicated the combined effect of nanoclay and gold nanoparticle loading in polyamide matrix. The char yield of neat polyamide was found lower i.e. 20%. However the char yield of the nanocomposite was increased with the nanofiller loading. From 0.1 to 0.5 wt.% MMT-GNP nanofiller loading, the char yield was increased from 37 to 60% [23, 24]. The 0.5 wt.% loading in PA/MMT/GNP 0.5 nanocomposite demonstrated greater increase in thermal properties signifying increased thermal stability. The comparison between TGA analyses of nanocomposite with 0.5% MMT/GNP and virgin polyamide showed that the thermal decomposition of the nanocomposites with clay and GNP initiated 108°C above the temperature of initial thermal decomposition of virgin polyamide.
|
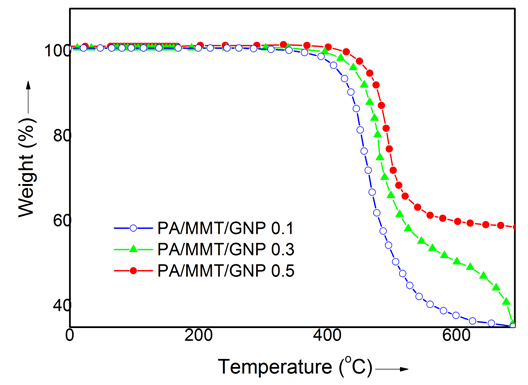 | Figure 4. TGA curves of PA/MMT/GNP nanocomposite at heating rate of 10 °C/min (N2) |
3.3. Flame-Retardant Properties
- To evaluate the flame retardancy, limiting Oxygen Index (LOI) values were recorded on a Stanton Redcroft flame meter by a modified method. LOI value of neat polyamide was found to be 24. The LOI value of PA/MMT/GNP 0.1 nanocomposite was high (40%), while the LOI value of 0.3 wt.% nanocomposite was further higher i.e. 55%. The highest LOI values were recorded for the 0.5 wt.% nano-filler loaded sample i.e. 63%. There appeared to be a correlation between the Limiting Oxygen Index and the nanofiller loading. The introduction of montmorillonite and gold nanoparticle conspicuously increased the flame retardancy of the nanocomposite while maintaining their thermal stability [25]. Thus, the influence of clay and GNP on nanocomposites was observed during flame retarding test, in which the burning rate was reduced by several % compared to virgin polyamide.
4. Conclusions
- An aromatic polyamide was synthesized and subjugated as a starting material for the preparation of nanocomposite. Two types of nanofiller were selected as reinforcement i.e. gold nanoparticle and montmorillonite clay. Later the polyamide/gold nanoparticle nanocomposite and polyamide/ montmorillonite/gold nanoparticle have been synthesized using simple route. These results manifestly demonstrated that the incorporation of MMT and gold nanoparticle enhanced the thermal properties as well as flame retardancy of processable aromatic polyamide. Novel polyamide can be considered as a candidate for the preparation of high-temperature resistant materials.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML