-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(2): 34-37
doi:10.5923/j.nn.20160602.03

Thermal Conductivity Measurement of Polyvinylpyrrolidone/Polyethylene/Graphene Nanocomposite
Ayesha Kausar
Nanosciences Division, National Centre for Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar , Nanosciences Division, National Centre for Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Thermal exfoliation of exfoliated graphite was employed as an efficient approach to produce single layer graphene. Two polymers were opted to be employed as thermally conducting matrices or to support the thermal conductivity of graphene i.e. polyvinylpyrrolidone (PVP) and polyethylene (PE). The PVP/PE nanocomposite with thermally exfoliated graphene were prepared using solution polymerization and precipitation technique. A series of PVP/PE nanocomposite was designed with varying nanofiller content of 0.1 to 0.5 wt.% graphene. The PVP/PE 0.1-0.5 nanocomposites were characterized by Fourier transform infrared spectroscopy (FTIR), thermal conductivity, and electrical conductivity measurement. FTIR analysis depicted characteristic absorption peaks for the formation of nanocomposite. The thermal conductivity of nanocomposite was significantly improved with graphene loading. The thermal conductivity of PVP/PE with 0.5 wt.% nanofiller was increased to 2.5 W/mK at higher temperature. The electrical conductivity of PVP/PE 0.1-0.5 was also found to increase in the range of 0.1-0.9 S/cm.
Keywords: Polyvinylpyrrolidone, Polyethylene, Grapheme, Electrical conductivity, Thermal conductivity
Cite this paper: Ayesha Kausar , Thermal Conductivity Measurement of Polyvinylpyrrolidone/Polyethylene/Graphene Nanocomposite, Nanoscience and Nanotechnology, Vol. 6 No. 2, 2016, pp. 34-37. doi: 10.5923/j.nn.20160602.03.
Article Outline
1. Introduction
- Over last twenty years, area of nanoscience has flourished and significance of nanotechnology has increased especially in the fields of sensors, computing, biomedical, and numerous other technical applications. In these disciplines, the developments are principally based on capability to synthesize nanoparticles of numerous materials, sizes and shapes, as well as to accumulate them into complex architectures. Due to their structural properties, recently nanomaterials have enormous range of applications. Moreover, scientists are investigating materials with enhanced physiochemical features that are dimensionally more appropriate in the area of nanoscience and technology. In this regard, discovery of graphene and graphene-based polymer nanocomposite is significant addition, thus playing an important role in modern science and technology [1]. Graphite has attracted remarkable attention as feasible and cheap filler for conducting nanocomposite [2, 3]. Derivatives of graphite with outstanding mechanical, thermal, and electrical features present an alternative to carbon nanotube. Various forms of nano-graphite such as exfoliated graphite (EG), expanded graphite, and graphene have been utilized to make conductive nanocomposite. According to research reports, the expanded graphite has strengthened the composite with thermosets and thermoplastics [4, 5]. Since the isolation by micromechanical cleavage of graphite, graphene has gained considerable attraction in numerous research fields [6-8]. Its exceptional atomically thin two-dimensional lattice structure fabricated with sp2-bonded carbon atoms have astonishing properties such as mechanical, electrical, thermal, and optical characteristics. It has shown several emergent applications in energy conversion and storage, electronics, photonics, catalysis, and polymer nanocomposites [9, 10]. For graphene-based material applications, stable, large-scale, high concentration dispersals of high-quality graphene in specific solvents are frequently desired. Thus far, a number of techniques have been established for graphene dispersal in numerous aqueous or organic solvents. Thermally and electrically conducting nanocomposites employing exfoliated graphite have been a chief attraction in current years. There are studies found on exfoliated graphite composites with diversity of polymers such as polystyrene (PS), poly (methyl methacrylate) (PMMA), high density polyethylene (HDPE), polypropylene (PP), polyaniline (PANI), Nylon, copolymers, and thermoset resins [11, 12]. Currently, graphene-based composite has fascinated both academic and industrial attention due to its remarkable features even at very low filler content. The graphene/graphene oxide modification and utilization in nanocomposite fabrication with diverse polymer matrixes have been explored. Wide range of techniques and various organic polymers have been utilized for the fabrication of graphene-filled polymer nanocomposites. The percolation threshold can be obtained at very low filler loading in case of modified graphene-based polymer nanocomposites. The preparation, structure, and features of polymer/graphene nanocomposites have been discussed in literature [13, 14]. Graphene-polymer composites have also been reported [15, 16]. For the nanofiller loading and interaction, polyethylene (PE) and polyvinylpyrrolidone (PVP) have been fascinating polymers. In polyvinylpyrrolidone, pyridyl group has strong efficiency for metals and has capability to undergo hydrogen bonding with polar species [17, 18]. In this attempt, graphene was prepared by the thermal exfoliation method. Two polymers were used for the formation of nanocomposite i.e. polyvinylpyrrolidone (PVP) and polyethylene (PE). The polymer blend was then reinforced with various graphene contents and tested for the thermal conductivity, thermal stability, and morphology analysis. To the best of our knowledge, polyvinylpyrrolidone/polyethylene/graphene (PVP/PE/G) nanocomposite have been prepared and studied for the first time in literature [19-22].
2. Experimental
2.1. Materials
- Graphite fine powder, polyvinylpyrrolidone (Mw 40,000), polyethylene (Mw~4,000), and N,N-dimethylformamide, anhydrous (DMF, 99.8%) were obtained from Aldrich.
2.2. Characterization Techniques
- IR spectra were taken at room temperature with a resolution of 4 cm-1 using Excalibur Series FTIR Spectrometer, Model No. FTSW 300 MX manufactured by BIO-RAD. Thermal conductivity of the samples was measured using a HotDisk Transient Plane Source TPS 2500 S (HotDisk AB). Electrical conductivity of the nanocomposite was measured using Zetasizer Nano (Malvern Instruments Ltd., Malvern, UK).
2.3. Preparation of Exfoliated Graphite
- Graphite powder was dispersed in DMF and ultra-sonicated for 24 h to prepare the exfoliated graphite. The exfoliated graphite was filtered and dried at 70°C [23].
2.4. Graphene Preparation (G)
- The thermal exfoliation of the exfoliated graphite was also carried out by placing 1g of powder in alumina boat. The boat was placed in quartz tube sealed at one end. The tube was inserted in furnace preheated at 900°C. The sample was kept in the furnace for 1 min. The graphite was reduced to form graphene [24].
2.5. Preparation of Polyvinylpyrrolidone/ polyethylene/graphene nanocomposite (PVP/PE/G) nanocomposite
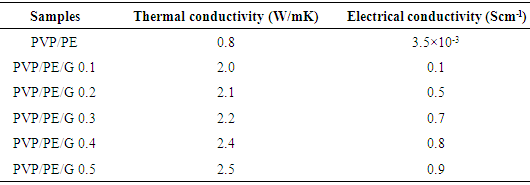
- Graphene (0.1-0.5 wt.%) was weighed and dispersed in DMF by sonication of 12 h. The PVP (10 w/v) solution was prepared in DMF with sonication of 6h. The PE (10 w/v) solution was also prepared in DMF with sonication of 6h. Later, both the polymer solutions were mixed and stirred for 2 h. The polymer mixture was then poured to the dispersed graphene mixture. The mixture was stirred for 2 h [25]. The nanocomposite mixture was then coagulated by adding water and the precipitated composites were dried in vacuum at 60°C (Fig. 1). FTIR (cm-1): 3001 (pyrrolidone ring C−H stretching vibration), 2955, 2901 (aliphatic C−H stretching vibration), 1726 (pyrrolidone C=O stretching vibration), 1271 (C−O stretching vibration), 1021 (C−C stretching vibration).
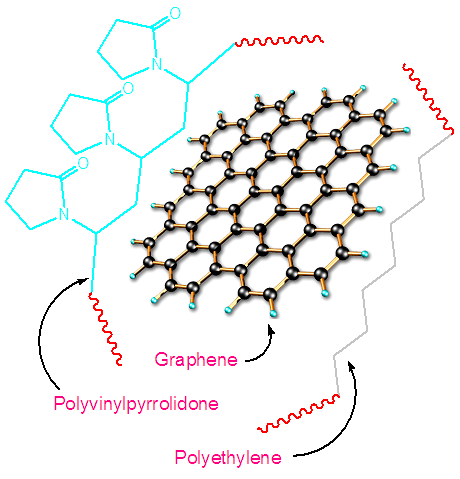 | Figure 1. Formation of PVP/PE/G nanocomposite |
3. Results and Discussion
3.1. Thermal Conductivity Measurement
- The graphene and its derived materials are found to be potentially significant in high-performance thermal systems [26, 27]. Several methods have been reported in literature measuring the thermal conductivity of materials. The thermal conductivity of nanocomposite has also been measured previously using transient hot-wire method. This method was also adopted in the present work to find out the thermal conductivity. In general, the thermal conductivity of nanoparticles greatly influences the thermal conductivity of the hybrid material. Therefore, graphene was thought to improve the thermal conductivity of PVP/PE/G 0.1-0.5 nanocomposite. In PVP/PE/G nanocomposite system, better interaction between polyvinylpyrrolidone/polyethylene copolymer and nanofiller enhanced the final properties of the nanocomposite. Fig. 2 and Table 1 show the linear dependence of thermal conductivity enhancement on temperature for PVP/PE/G 0.1-0.5 nanocomposite. There was ascending trend in the thermograms demonstrating better performance at high temperature. According to the results, the thermal conductivity was found to enhance with graphene loading in copolymer matrix. The PVP/PE/G 0.5 nanocomposite has shown better properties than PVP/PE/G 0.1 nanocomposite. The PVP/PE/G 0.5 revealed higher thermal conductivity value of 2.5 W/mK at higher temperature. The thermal conductivity of PVP/PE/G 0.1 with 0.1 wt.% nanofiller was increased to 2.0 W/mK at higher temperature. Hence it was accomplished that the increase in graphene content improved the thermal conductivity of nanocomposite compared with the neat PVP/PE copolymer having the value of 0.8 W/mK.
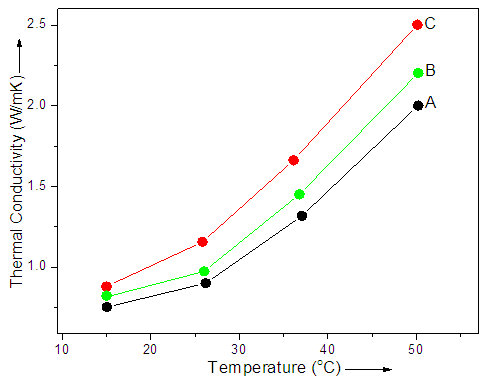 | Figure 2. Thermal conductivity of (A) PVP/PE/G 0.1; (B) PVP/PE/G 0.3; and (C) PVP/PE/G 0.5 nanocomposite |
|
3.2. Electrical Conductivity Analysis
- Electrical conductivity of graphene-based nanocomposite has been less explored in literature relative to the thermal conductivity measurement. Generally the electrical conductivity may increase or decrease depending upon nanofiller loading level. The electrical conductivity of the nanocomposite was measured at 25°C, and the results are presented in Fig. 3 (Table 1). For PVP/PE/G 0.1-0.5 nanocomposite, the electrical conductivity was slightly increased with the nanofiller loading. Thus the electrical conductivity was found to vary from 0.1 S/cm (0.1 wt.% graphene) to 0.9 S/cm (0.5 wt.% graphene). The electrical conductivity of neat copolymer was however very low i.e. 3.5×10-3 S/cm. Moreover after 0.5 wt.% nanofiller loading, decrease in electrical conductivity was expected due to the nanofiller aggregation. Therefore the nanofiller loading was adjusted between 0.1 to 0.5 wt.%. In other words, 0.1 to 0.5 wt.% graphene was opted as optimum nanofiller loading. The electrical conductivity was also enhanced compared with the reported polymer/graphene nanocomposite [28, 29].
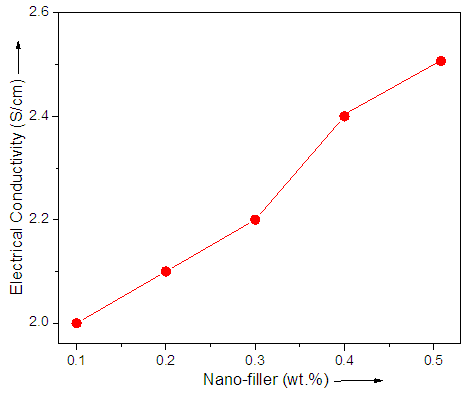 | Figure 3. Electrical conductivity of PVP/PE/G nanocomposite |
4. Conclusions
- This investigation was about the design, structure, thermal, and electrical conductivity of the nanocomposite. Effect of the addition of graphene in the copolymer of polyvinylpyrrolidone/polyethylene has been studied. The nanofiller was encumbered in varying content to perceive the effect of concentration on the physical properties. Formation of copolymer matrix increased the stability and homogeneity of the nanofiller in the nanocomposite and prohibited its aggregation. According to thermal conductivity measurement, increasing temperature and concentration led to increase in thermal conductivity of nanofluids. The maximum enhancement in thermal conductivity was obtained for 0.5 wt.% garphene loading. Similarly the electrical conductivity for PVP/PE/G 0.1-0.5 nanocomposite was found to increase from 0.1 to 0.9 S/cm.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML