| [1] | Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV and Firsov AA, 2004,. Electric Field Effect in Atomically Thin Carbon Films. Science. 306, 666-669. |
| [2] | Sakhaee-Pour A, Ahmadian MT, Vafai A, 2008, Applications of single-layered graphene sheets as mass sensors and Atomistic dust detectors. Solid State Communications. 145, 168-172. |
| [3] | Stoller MD, Park S, Zhu Y, An J and Ruoff RS. 2008, Graphene-Based Ultracapacitors. Nano Lett, 8, 3498–3502. |
| [4] | Choi SH, Kim YL, Byun KM. 2011, Graphene-on-silver substrates for sensitive surface plasmon resonance imaging biosensors. Opt Express. 19, 458-466. |
| [5] | Peigney A, Laurent Ch, Flahaut E, Bacsa RR, Rousset A. 2001, Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon. 39, 507-514. |
| [6] | Kang X, Wang J, Wu H, Aksay IA, Liu J and Lin Y. 2009, Glucose Oxidase–graphene–chitosan modified electrode for Direct electrochemistry and glucose sensing Biosens. Bioelectron. 25, 901-905. |
| [7] | Liu Y, Yu D, Zeng C, Miao Z and Dai L. 2010, Biocompatible Graphene Oxide-Based Glucose Biosensors. Langmuir, 26, 6158-6160. |
| [8] | Zhuang Z, Li J, Xu R, Dan X. 2011,Electrochemical Detection of Dopamine in the Presence of Ascorbic Acid Using Overoxidized Polypyrrole/Graphene Modified Electrodes. J. Electrochem. Sci. 6, 2149 – 2161. |
| [9] | Du D, Zou Z, Shin Y, Wang J, Wu H, Engelhard MH, Liu J, Aksay IA, Lin Y. 2010, Sensitive Immunosensor for Cancer Biomarker Based on Dual Signal Amplification Strategy of Graphene Sheets and Multienzyme Functionalized Carbon Nanospheres. Anal. Chem. 82, 2989-2995. |
| [10] | Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lina Y. Graphene Based Electrochemical Sensors and Biosensors. Electroanalysis 22, 1027 – 1036. |
| [11] | Dan P, Lu Y, Kybert NJ, Luo ZT, Johnson ATC. 2009, Intrinsic Response of Graphene Vapor Sensors. Nano Letters. 9, 1472-1475. |
| [12] | Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, Novoselov KS. 2007, Detection of individual gas molecules adsorbed on graphene. Nature Materials. 6, 652 – 655. |
| [13] | Ang PK, Chen W, Wee ATS, Loh KP. 2008, Solution-gated epitaxial graphene as pH sensor. J. Am. Chem. Soc. 130, 14392–14393. |
| [14] | Dong X, Shi Y, Huang W, Chen P, Li LP. 2010, Electrical Detection of DNA Hybridization with Single-Base Specificity Using Transistors Based on CVD-Grown Graphene Sheets. Adv. Mater. 22, 1–5. |
| [15] | Mohanty N, Berry V. 2008, Graphene-based single-bacterium resolution biodevice and DNA transistor: interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Letters. 8, 4469–4476. |
| [16] | Ohno Y, Maehashi K, Yamashiro Y, Matsumoto K. 2009, Electrolyte-gated graphene field-effect transistors for detecting pH and protein adsorption. Nano Letters. 9, 3318–3322. |
| [17] | Yang W, Ratinac KR, Ringer SP, Thordarson P, Gooding JJ, Braet F. 2010, Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene? Angew. Chem. Int. Ed. 49, 2114 – 2138. |
| [18] | Wang Y, Li Z, Wang J, Li J, Lin Y. Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends in Biotechnology. 2011, 29, 205-212. |
| [19] | Hirsch A, Vostrowsky O. Functionalization of Carbon Nanotubes. Topics in Current Chemistry. 2005, 45, 193-237 |
| [20] | Martinez MT, Tseng YC, Ormategui N, Loinaz I, Eritja R, Bokor . 2009, J. Label-Free DNA Biosensors Based on Functionalized Carbon Nanotube Field Effect Transistors. Nano Letters 9, 530–536. |
| [21] | Jeng ES, Moll AE, Roy AC, Gastala JB, Strano MS. 2006, Detection of DNA Hybridization Using the Near-Infrared Band-Gap Fluorescence of Single-Walled Carbon Nanotubes. Nano Letters, 6, 371–375. |
| [22] | Lu C-H, Yang H-H, Zhu CL, Chen X, Guo-Nan Chen Prof. 2009, A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed, 48, 4785–4787. |
| [23] | Balavoine F, Schultz P, Richard C, Mallouh V, Ebbesen TW, Mioskowski C. 1999, Helical crystallization of proteins on carbon nanotubes: a first step towards the development of new biosensors. Angew. Chem. Int. Ed38, 1912–1915. |
| [24] | Loh KP, Bao Q, Ang PK, Yang. 2010, The chemistry of graphene. J. Mater. Chem20, 2277–2289. |
| [25] | Leyden MR, Schuman C, Sharf T, Kevek J, Remcho VT, Minot ED. 2010, Fabrication and Characterization of Carbon Nanotube Field-Effect Transistor Biosensors. Proc. SPIE, doi:10.1117/12.861329. |
| [26] | Balasubramanian K. Burghard M. 2005, Chemically functionalized carbon nanotubes. Small, 1, 180-192. |
| [27] | Dreyer DR, Park S, Bielawski CW and Ruoff RS. 2010, The chemistry of graphene oxide. Chem. Soc. Rev.39, 228-240. |
| [28] | Balasubramanian K, Burghard M. 2008, Electrochemically functionalized carbon nanotubes for device applications. J.Mater. Chem. 18, 3071-3083. |
| [29] | Maroto A, Balasubramanian K, Burghard M, Kern K. 2007, Functionalized metallic carbon nanotube devices for pH sensing. ChemPhysChem. 8, 220-223. |
| [30] | Balasubramanian K, Friedrich M, Jiang C, Fan Y, Mews A, Burghard M, Kern K. 2003, Electrical transport and confocal Raman studies of electrochemically modified individual carbon nanotubes. Adv. Mater. 15, 1515-1518. |
| [31] | Currie, Janet, Wanchuan Lin, Wei Zhang, 2011. Patient Knowledge and Antibiotic Abuse: Evidence from an Audit Study in China. Journal of Health Economics 30, 933-949. |
| [32] | Dar-Odeh, Najla Saeed, Abu-Hammad Osama Abdalla, Al-Omiri, Mahmoud Khaled, Khraisat, Ameen Sameh, Shehabi, Asem Ata, 2010. Antibiotic Prescribing Practices by Dentists: a Review. Therapeutics and Clinic Risk Management 6, 301-306. |
| [33] | Mendham, J.; Denny, R.C.; Barnes, J.D.; Thomas, M (2000). Vogel's textbook of quantitative chemical analysis (5th.ed.). Harlow: Pearson Education. |
| [34] | Eggleston, Karen, Ling Li, Qingyue Meng, Lindelow Magnus, Wagstaff Adam, 2008. Health Service Delivery in China: A Literature Review. Health Economics 17(2), 149-165. |
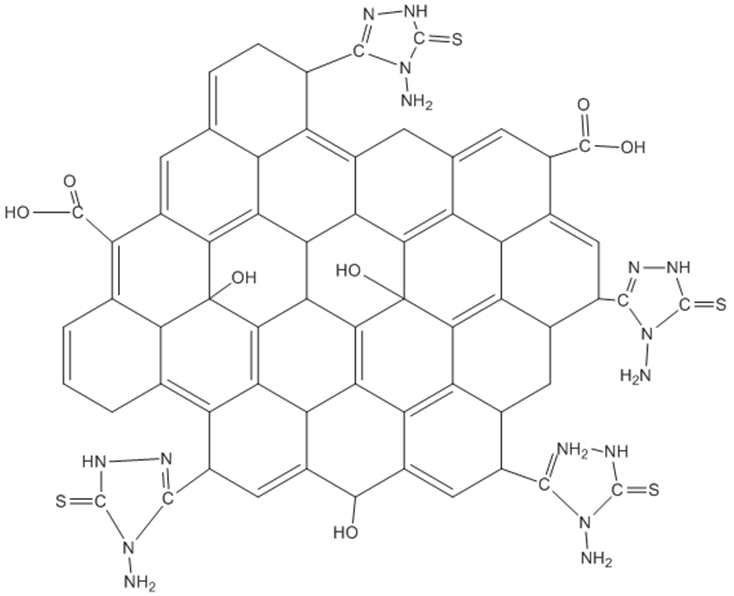
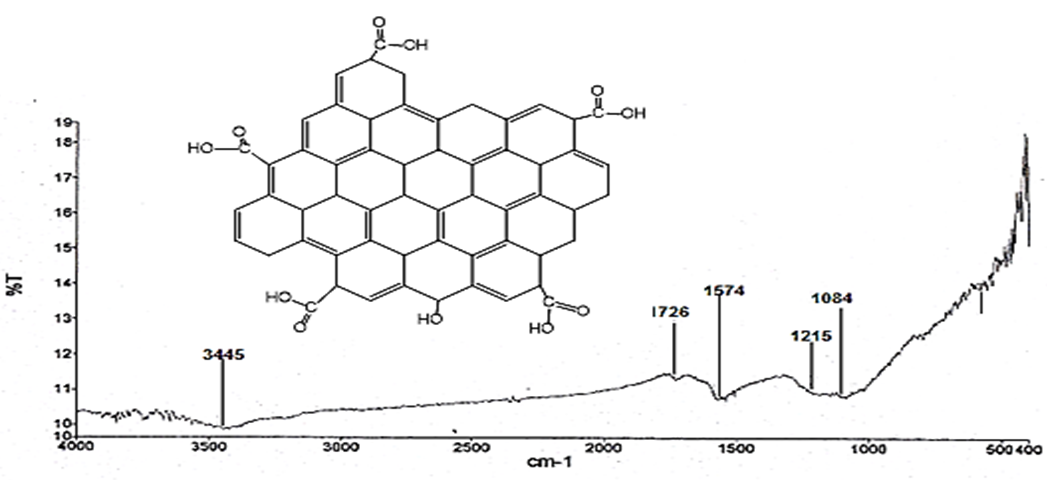
| [35] | I. Jung, (2007), Simple approach for high –contrast optical imaging and characterization of graphene based sheets. Nano Lett.7 3569-3575. |
| [36] | CK. Chua and M. Pumera. (2014), Chemical reduction of graphene oxide: a synthetic chemistry viewpoint, Chem. Soc. Rev. 43 291-312. |
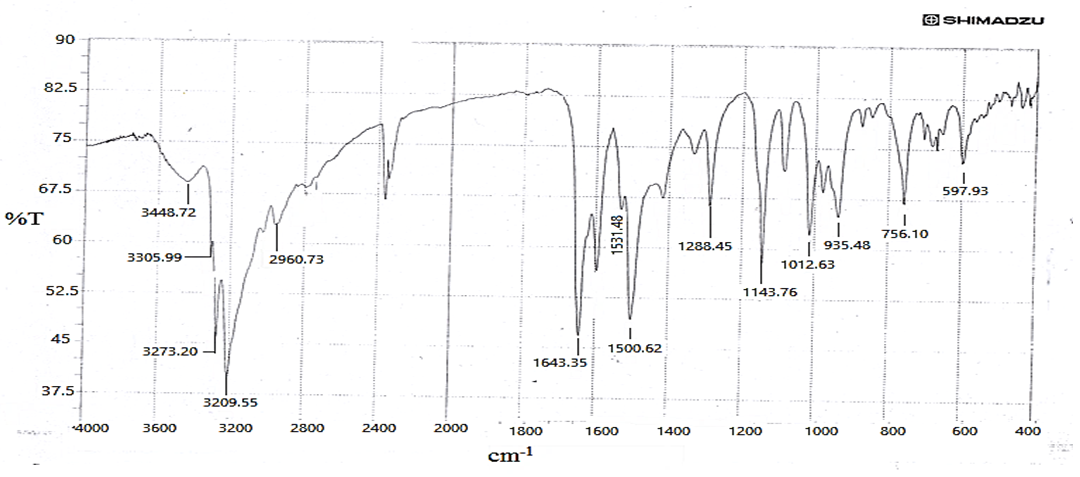
| [37] | XH. Sun and YF Liu, study on the synthesis of thiocarbohydrazide chemistry (china) (1999) 62:46-48. |
| [38] | A. Vasilescu, S. Andreescu, C. Bala, S.C. Litescu, T. Noguer, J.L. Marty, (2003), Screen printed electrodes with electropolymerized Meldola. Blue as versatile detectors in biosensors, Biosensors and Bioelectronics. 18, 781–790. |
| [39] | Devesh K. M., Subhendu B., Mostafaizur R. Dipak K. "Morphology and Cyclic Voltammetry Analysis of in situ Polymerized Polyaniline /Graphene Composites" J. Electrochem. Sci.Eng., Vol.3 ,no.4,pp.157-166, 2013. |
| [40] | I. Latif, Taghreed B. Alwan, Ammar H. 2012, Low Frequency Dielectric Study of PAPA-PVA-GR Nanocomposites 2(6): 190-200. |
| [41] | GR. Burns, (1968) metal complexes of thiocarbohydrazide'' Inorg. Com.7:277-283. |
| [42] | A. Gomis-Berenguer, M. Go´mez-Mingot, V. Montiel, A. Canals, T. Thiemann, R. O. Kadara, C. E. Banks and J. Iniesta, 2012, RSC Adv., 2, 7735–7742. |
| [43] | M.E. Swatz, I.S Krull. Analytical Method Development and Validation, Marcel Dekker, New York; 1997. |
| [44] | M. A. Khalilzadeh, F. Khaleghi, F. Gholami & H. Karimi- Maleh." Electrocatalytic Determination of Ampicillin Using Carbon-Paste Electrode Modified with Ferrocendicarboxylic Acid" J. Analy. LettersVolume 42, Issue 3, 2009, pages 584-599. |
| [45] | Kloth, K.; Rye-Johnsen, M.; Didier, A.; Dietrich, R.; Martlbauer, E.; Niessner, R.; Seidel, M. A, 2009, regenerable immunochip for the rapid determination of 13 different antibiotics in raw milk. Analyst 134, 1433–1439. |
| [46] | Adrian, J.; Pinacho, D.G.; Granier, B.; Diserens, J.M.; Sanchez-Baeza, F.; Marco, M.P. A 2008, multianalyte ELISA for immunochemical screening of sulfonamide, fluoroquinolone and beta-lactam antibiotics in milk samples using class-selective bioreceptors. Anal. Bioanal. Chem. 391, 1703–1712. |



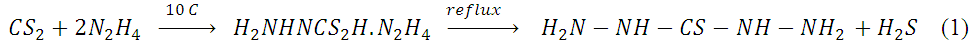
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML