-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(2): 17-23
doi:10.5923/j.nn.20160602.01

Preparation and Characterization of ZnO/polystyrene Nanocomposite Films Using Ultrasound Irradiation
Alwan N. Jassim 1, Riyadh M. Alwan 1, Quraish A. Kadhim 2, Ahmed A. Nsaif 3
1Corporation of Research and Industrial Development, Iraqi Ministry of Industry and Minerals, Iraq
2Chemical and Petrochemical Research Center, Corporation of Research and Industrial Development, Iraqi Ministry of Industry and Minerals, Iraq
3Environmental Research Center, University of Technology, Ministry of High Education Baghdad, Iraq
Correspondence to: Alwan N. Jassim , Corporation of Research and Industrial Development, Iraqi Ministry of Industry and Minerals, Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the present work, thin films containing nano zinc oxide and polystyrene were prepared via sol-gel process followed by film casting with 5 wt% concentration of ZnO. The prepared nano composite films were characterized using atomic force microscopy (AFM), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) analysis, differential scanning calorimeter (DSC) and UV-Vis analysis. Atomic force microscopy showed that ZnO nano particles were homogeneously dispersed in the polystyrene matrix, and root mean square (RMS) was 5.24 nm for neat polystyrene and found to be 6.2 nm for ZnO/polystyrene composite film. The introduction of ZnO nano particles increased the glass transition temperature by 6.81°C. The transmittance and absorbance of UV-Vis light exhibited normal spectra for these films. The X-ray diffraction pattern verified the hexagonal structure of nano ZnO and FT-IR spectra evidenced the existence of ZnO nano particles and polystyrene in ZnO/Ps composite film.
Keywords: Sol-gel, ZnO nanoperticles, Polystyrene, AFM, XRD, DSC, FT-IR and UV-Vis
Cite this paper: Alwan N. Jassim , Riyadh M. Alwan , Quraish A. Kadhim , Ahmed A. Nsaif , Preparation and Characterization of ZnO/polystyrene Nanocomposite Films Using Ultrasound Irradiation, Nanoscience and Nanotechnology, Vol. 6 No. 2, 2016, pp. 17-23. doi: 10.5923/j.nn.20160602.01.
Article Outline
1. Introduction
- Nowdays, a lot of work has been done on organic/ inorganic nanocomposite films. A combination of organic and inorganic materials results in the formation of composites that show the properties of both components, creating wide scope of applications in science and technology. Besides, improvements of many properties are achieved at a very low loading of nanoinorganic materials, compared to micro-sized fillers [1-6].Among many inorganic materials, ZnO is specifically interesting owing to its variety of application fields like solar cells, gas sensors, Varistors, luminescent devices and antibacterial activity. When the size of ZnO crystal decreases to nano scale, it can have mechanical, optical, electrical, thermal properties and antibacterial activity quite different from the bulk [7-16]. Nano particles are much more reactive than larger particles because of their small size and large surface area. They have many advantages like high surface to volume ratio, good chemical, biocompatibility and easy fabrication which makes them appropriate for preparation hygienic surfaces [17-24]. The polymer matrix composites are very important as they are most widely used because of their lightness, ease of fabrication and a variety of other properties [25-31]. ZnO nanoparticles can be prepared easily via sol-gel or wetchemical method, thermal decomposition and chemical vapor route [32-37].A great deal of research has been focused on the development of ZnO/polymer nano composite materials using different polymer systems. Polystyrene polymer is transparent thermoplastic material, with lots of prospects for making composite material with nano structured ZnO. Introduction of ZnO filler into polymeric matrices can modify their optical, electrical and other properties [38-44].The present work is a simple attempt to prepare and characterize of ZnO/polystyrene nano composite thin films via mixing and casting process.
2. Experimental Section
- All chemicals were purchased from a Merck company and used without further purification. The solutions were prepared by using distilled water. Preparation of nano zinc oxide are illustrated else where [45]. In brief, gel of zinc oxide was prepared as follows: 12.6g of zinc acetate dihydrate was dissolved in 400ml of distilled water, then 600ml of ethanol was added slowly at 50°C, and 6ml of H2O2 (47%) was added dropwise then mixed for one hour. Then, this solution was placed in an ultrasonic vessel with rated output power of 750 W and frequency 24 kHz, to get clear solution and keptuntil use. Sonics vibra cell from USA sonics and materials, INC company was used in this experment. For comparison purpose, we prepared nano zinc oxide powder by drying part of the above solution in oven for several hours at 80°C.To prepare ZnO/polystyrene nano composite film, in a typical experiment, 2g of polystyrene (Sabic company) was dissolved in 50ml of toluene and then directly added into the prepared gel of ZnO. The concentration of ZnO was taken as 5 wt% to polystyrene. The solution mixture was then poured into 10x15 cm clean and dry glass mold. The solvents were evaporated slowly in a dust free chamber at room temperature, then composite films were obtained after evaporation and then heated for several hours at 80°C to remove any solvents and to convert zinc gel into zinc oxide nano particles. Also, neat polystyrene film without nano material was prepared similarly to this procedure with similar thicknesses.
3. Results and Discussion
- The prepared composite thin films were flexible crack free and the achievement of transparency can be readily seen by nacked eyes. Figure 1 shows the photograph image of the film cast from toluene with 130 µm thickness in average.
 | Figure 1. Photograph of the nanocomposite film prepared by casting. The thickness of the film is around 130 µm. The amout of ZnO was fixed to 5 wt% |
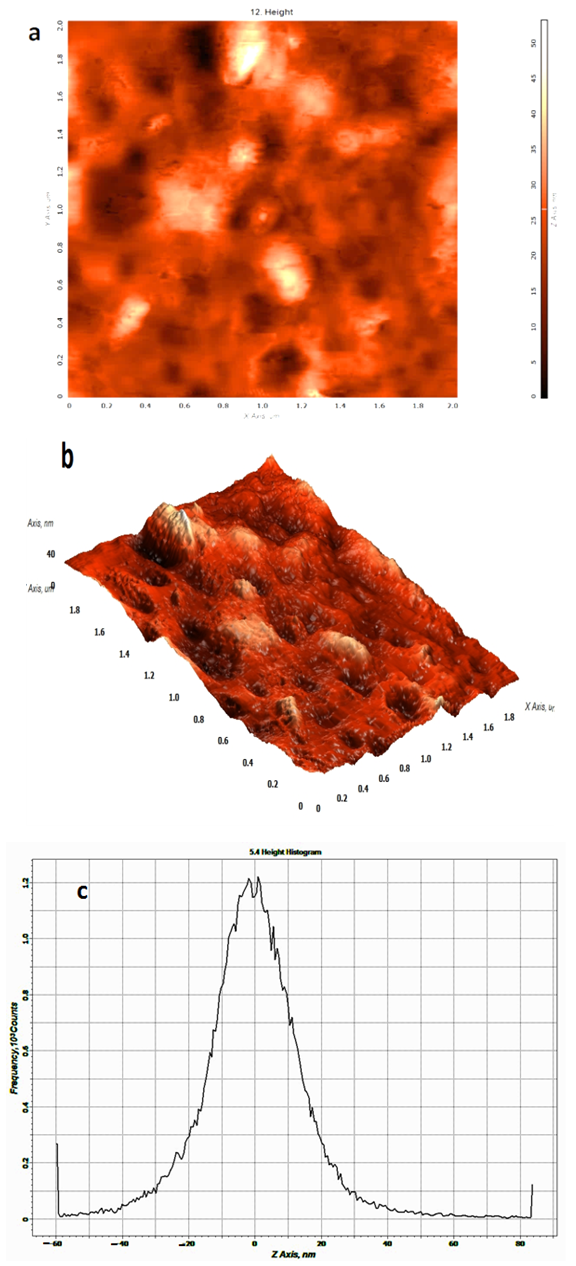 | Figure 2. AFM 2-D image (a) and its corresponding 3-D image (b) of the ZnO/PS nano composite film in a scan area of 2X2 µm, and (c) shows good dispersion of nanoparticles in PS matrix |
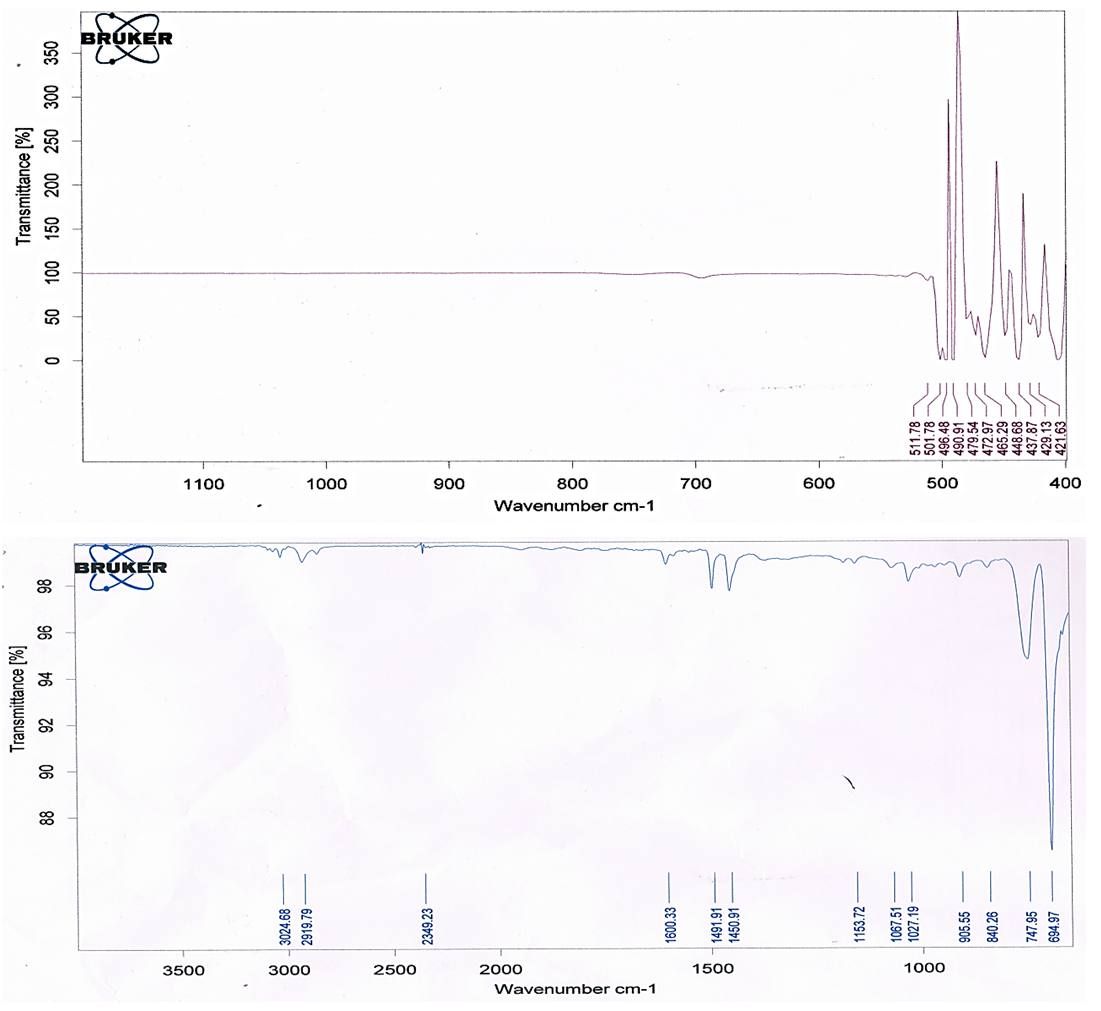 | Figure 3. FT-IR transmission spectra of ZnO/PS nanocomposite film |
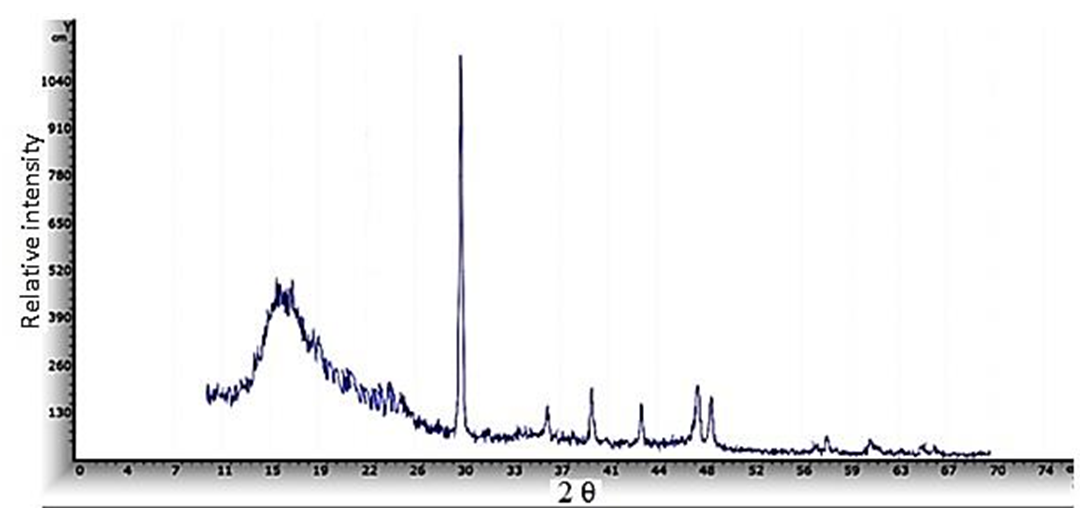 | Figure 4. XRD pattern of ZnO/PS nanocompsite film prepared at room temperature |
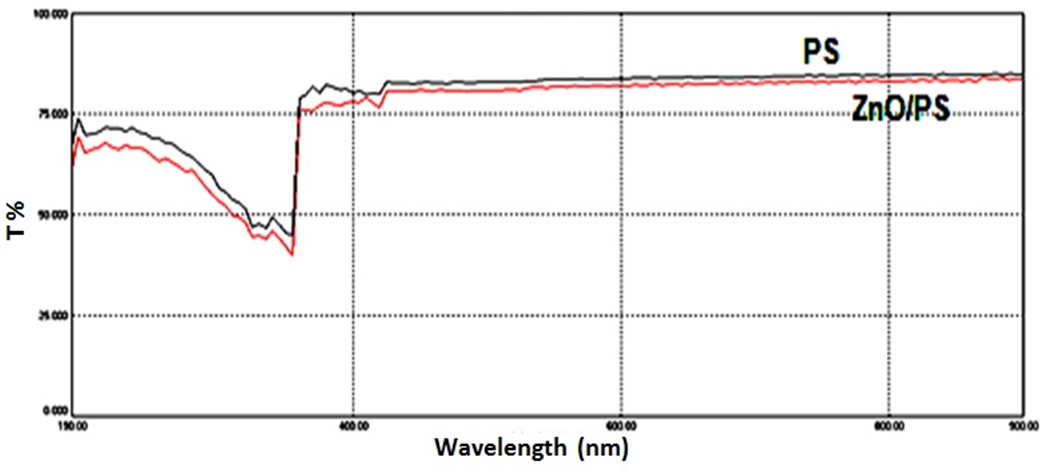 | Figure 5. UV-Vis transmission spectra of the ZnO/PS nanocomposite film, and of plain polystyrene film |
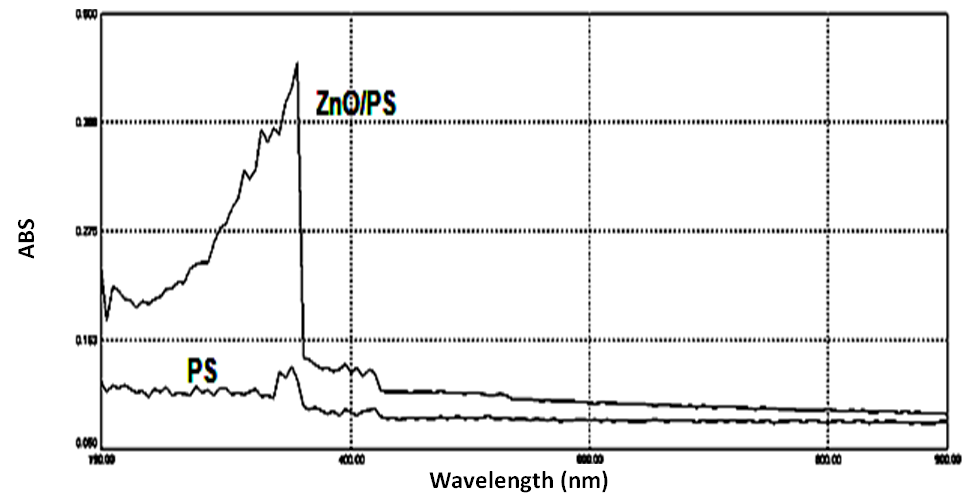 | Figure 6. UV-Vis absorption spectra of ZnO/PS film and PS film at room temperature |
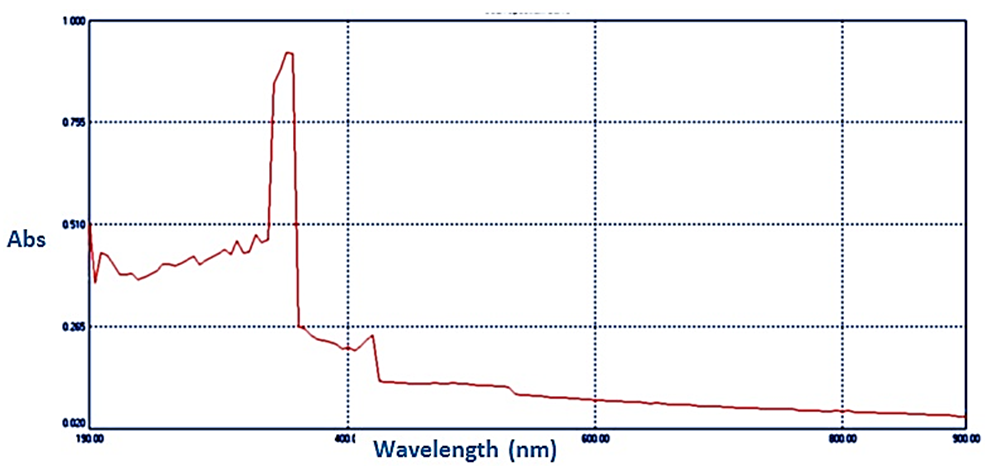 | Figure 7. Absorption spectrum of ZnO gel |
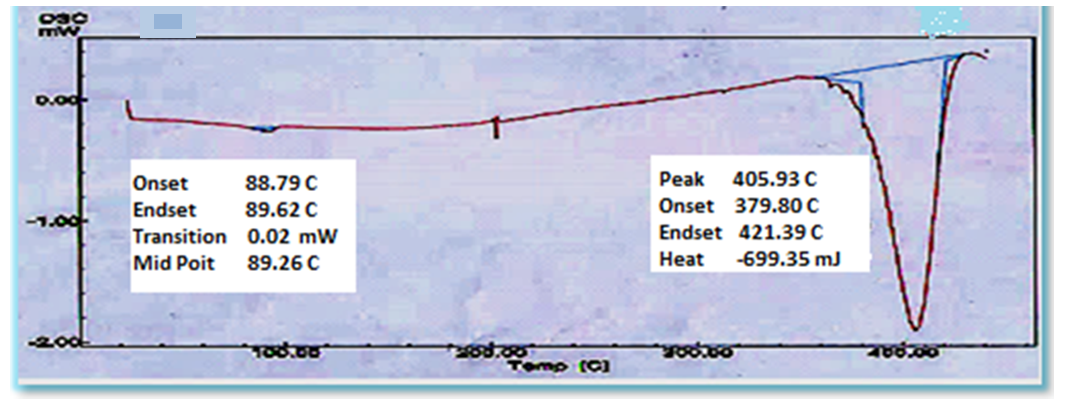 | Figure 8. DSC thermogram of ZnO/PS nanocomposite film |
4. Conclusions
- We have demonstrated the synthesis of ZnO/polystyrene nanocomposite film with 5% of ZnO through the mixing process. The nano zinc oxide improved the thermal properties of the prepared composite film and increased the root mean square of their surfaces, beside a dramatic change in the absorption intensity in the UV region. Also, the XRD, FT-IR and UV-Vis analyses exhibited the high purity of the prepared nanocomposite film. Further work remains to be done on various other thin film combination uesing different materials.
5. Funding / Support
- The authors wish to extend their gratitude to the Iraq ministry of industry and minerals for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML