-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1): 6-10
doi:10.5923/j.nn.20160601.02

Thermal and Rheological Properties of Waterborne Polyurethane/Nanodiamond Composite
Ayesha Kausar
Nanosciences Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this paper, waterborne polyurethane (WPU) and its nanocomposite with nanodiamond (WPU/ND) has been synthesized using facile route. The morphology properties with various nanodiamond content were investigated using scanning electron microscopic (SEM). The microphotographs confirmed the covalent interaction between waterborne polyurethane and ND via better dispersion. The thermal properties studied by thermogravimetric analysis showed the improvement in the maximum degradation temperature up to 501°C. Rheological tests showed increased melt viscosity with functional ND loading due to better WPU/ND interaction, whereas nanodiamond loading reduced the variation of processing viscosity.
Keywords: Waterborne polyurethane, Nanodiamond, Rheology, Thermal properties
Cite this paper: Ayesha Kausar, Thermal and Rheological Properties of Waterborne Polyurethane/Nanodiamond Composite, Nanoscience and Nanotechnology, Vol. 6 No. 1, 2016, pp. 6-10. doi: 10.5923/j.nn.20160601.02.
Article Outline
1. Introduction
- One of the most fascinating synthetic polymers is polyurethane (PU). Because of its distinctive features, more interest has been paying attention to the morphology, synthesis, chemical, and mechanical characteristics [1, 2]. The linear arrangement of segmented PU appears in the form of (A–B)n. The soft-segment is generally polyether macrogel of molecular weight ranges between 1000 and 3000. The hard-segment is usually comprised of low-molecular weight diamine or diol react with diisocyanate. Due to the chemical structure difference, the hard and soft segment form micro-domains by joint attraction linking intermolecular hydrogen bonding. For waterborne polyurethane (WPU) systems, during the drying process, only water was evaporated. Thus interpenetrating these systems with WPU are safe with reference to the environment. They are non-flammable, non-toxic and do not create polluted air or waste water [3-5]. Carbon nanotube (CNT) generates a huge prospective in polymer composite synthesis because of its axial tensile strength. In addition to the outstanding mechanical features, CNT exhibits advanced electrical and thermal properties such as thermal conductivity about twice as high as diamond, high thermal stability up to 2800°C in vacuum. Moreover, it is 1000 times superior to copper wires in electric-current-carrying capacity [6-8]. The CNT-based composites have been widely studied by employing different polymer matrix materials [9-12]. Single-walled carbon nanotube (SWCNT) strengthened polyurethane composite membranes were reported. In the low stress region (0-2 MPa) and plastic deformation at higher stress, all the membranes display a nonlinear elastic behavior [13, 14]. The tensile strength of PU/SWCNT composites was improved by 46% relative to pristine polymer [15, 16]. Other distinctive filler is nanodiamond (ND). In comparison to carbon nanotube and other carbon nanoparticles, ND has a large number of different functional groups, terminating the surface of particle [17-19]. The surface functional groups layer is an essential property of this material. Surface chemistry of ND is prosperous and surface functionalization of ND can be obtained to any degree without compromising the valuable features of the diamond core. Consequently, the capability to adapt and control the surface chemistry of ND is of greatest consequence [19, 20]. The small ~5 nm diameter of discharge ND particles in combination with their greater and exceptional mechanical properties (diamond properties), and rich surface chemistry, makes ND the ideal candidate for strengthening polymer matrices. Where it can act through altering the properties of the inter-phase as well as generating a strong covalent interface with the matrix nanodiamond (ND) can be considered of as a widespread building block for polymer nanocomposite design. Since its surface can be tailored to almost any degree in order to generate advantageous interfaces and affect the inter-phase with virtually any polymer matrix without compromising the properties of the diamond core [21]. A conspicuous contrast to CNT and other sp2 carbon nanostructures in which surface functionalization frequently results in degradation of their properties, ND show better medication properties [22]. Therefore development in the polymer/ND design been focused and the thermal and rheological properties of these composites have been described in literature [23]. In this study, waterborne polyurethane/nanodiamond nanocomposite was prepared. Chemical fictionalization of nanodiamond was used to improve the compatibility between ND and waterborne polyurethane. Thermal and rheological properties of the composite have been characterized.
2. Experimental
2.1. Chemicals
- Diamond nanopowder (<10 nm particle size, ≥97%), polycaprolactone-block-polytetrahydrofuran-block-poly-caprolactone average (Mn ~ 2,000), isophorone diisocyanate (IPD), and triethylamine (TEA) were purchased from Aldrich, and were used as received without further purification.
2.2. Instrumentation
- IR spectra were taken at room temperature using Excalibur Series FTIR Spectrometer, Model No. FTSW 300 MX manufactured by BIO-RAD. Thermal stability was determined by METTLER TOLEDO TGA/SDTA 851 thermogravimetric analyzer using 1-5 mg of the sample in Al2O3 crucible at a heating rate of 10°C/min under nitrogen atmosphere. The scanning electron microscopic (SEM) images were obtained by Scanning Electron Microscope S-4700 (Japan Hitachi Co. Ltd.). Rheological properties were studied using a parallel plate rheometer (ARES Rheometric Scientific Rheometer, USA) in the range 25 to 200°C (temp rate = 3°C/min; strain = 1; frequency = 10 Hz).
2.3. Acid Treatment of Nanodiamond
- Nanodiamond carboxylation was performed by treating the nanodiamond with strong acids i.e. H2SO4 and HNO3 (3:1) with continuous stirring of 24 h (room temperature). The above mixture was poured into 500 mL water and again stirred for 4 h (Fig. 1). After filtration, the residue was washed several times with deionized water and dried at 80°C for 4 h [24]. FTIR: 3479 (hydroxyl group), 3003 (aromatic C–H stretch), 1720 (C=O).
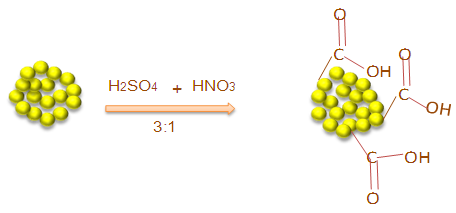 | Figure 1. Acid functionalization of nanodiamond |
2.4. Synthesis of WPU
- Polycaprolactone-block-polytetrahydrofuran-block-polycaprolactone (40 g) was heated to 80°C in a four-necked flask equipped with a mechanical stirrer, condenser, and thermometer. After complete melting of the polyol, 20 g IPD was added drop wise to the reaction mixture under nitrogen atmosphere. Afterwards 100 mL of acetone was poured into the flask. The mixture was then cooled to 60°C. After the addition of 3g TEA, the product was dispersed in 200 mL distilled water with vigorous stirring. The solid was extracted using rotary vacuum evaporating at 50°C. FTIR: 3311 cm-1 (N–H stretch), 1590 cm-1 (N–H bend), 3001 cm-1 (aromatic C–H stretch), 2890 cm-1 (aliphatic C–H stretch), 1720 cm-1 (urethane C=O stretch), 1654 cm-1 (amide C=O stretch), 1230 cm-1 (C–O stretch).
2.5. Preparation of WPU/ND Nanocomposite Film
- To obtain composite films, WPU were mixed with various content of filler suspension. After mixing for 4 h, the resulting mixtures were cast in Petri dishes and dried at 50°C for 10-20 h. By changing the filler content over the range of 1-10 wt. %, a series of nanocomposite films were prepared with a thickness of ~0.2 mm (Fig. 2).
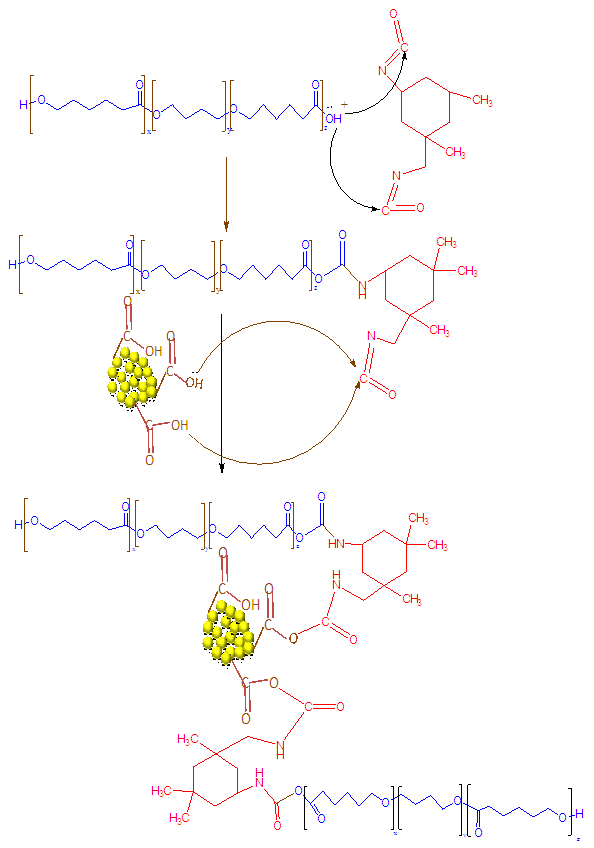 | Figure 2. Interaction between nanodiamond and waterborne polyurethane |
3. Results and Discussion
3.1. Morphological Analysis
- The morphology and dispersion of WPU/ND composite was examined using SEM. Fig. 3A-C shows high magnification SEM images of the fractured surface morphology. No large ND clusters were found on the fractured surface. In all nanocomposites, ND dispersion was also homogeneous throughout the WPU matrix. The functional ND was seemed to be embedded in WPU. The results indicated the existence of strong interfacial bonding i.e. covalent bonding between functional nanodiamond and WPU. Furthermore, the quality of ND dispersion in polyurethane matrix directly correlates with the efficiency for improving thermal, and other physical properties.
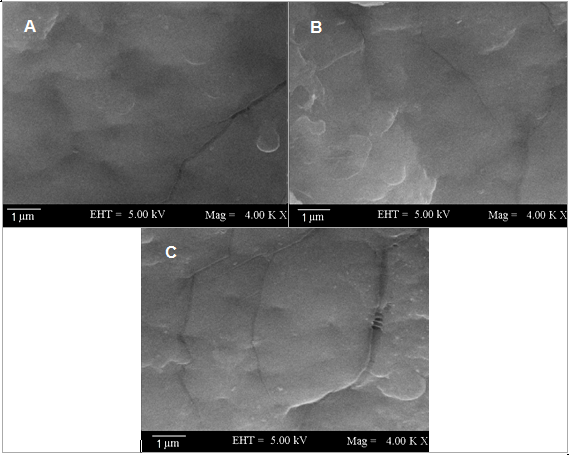 | Figure 3. SEM micrographs of (A) WPU/ND 1; (B) WPU/ND 5; and (C) WPU/ND 10 |
3.2. Thermal Degradation of WPU/ND Nanocomposite
- Fig. 4 presents the thermal degradation behavior of waterborne polyurethane and nanodiamond nanocomposite with various ND content. Results show that the initial thermal degradation temperature of WPU/ND 1-10 nanocomposite was in the range of 279 to 331°C. The maximum thermal degradation temperature of WPU/ND 1-10 nanocomposite was around 377 to 501°C. The results indicated that the nanodiamond addition rise the thermal degradation temperature of the composites, similar to CNT [25-27].
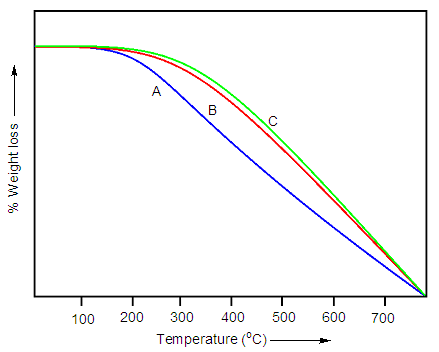 | Figure 4. Thermal degradation curves of (A) WPU/ND 1; (B) WPU/ND 5; and (C) WPU/ND 10 |
3.3. Rheological Properties of WPU/ND
- Influence of nanodiamond addition on rheological properties of the composites was also studied. Addition of ND increased the melt viscosity of nanocomposite, as shown in Fig. 5. The melt viscosity of WPU/ND 10 nanocomposite was higher than that of WPU/ND 1 nanocomposite. From the results of rheological test, the viscosity variation was reduced by ND inclusion indicating that the ND can stabilize the viscosity of polymer melting. Moreover higher processing temperature (beyond 200°C) may destroy the interface between waterborne polyurethane and ND. This may result in the phase segregation in WPU/ND nanocomposite.
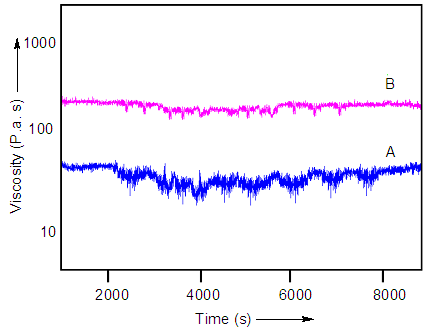 | Figure 5. Viscosity of nanocomposite versus time for (A) WPU/ND 1; and (B) WPU/ND 10 |
4. Conclusions
- In this attempt, waterborne polyurethane nanocomposite with nanodiamond has been effectively prepared. The thermal properties showed that the addition of ND improved the thermal degradation properties of nanocomposite with filler loading of 1-10 wt. %. SEM microphotographs show that the ND can be efficiently dispersed via covalent bond formation between waterborne polyurethane and ND. Rheological tests showed that the nanofiller increased the melt viscosity and reduced the variation of processing viscosity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML