-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2016; 6(1): 1-5
doi:10.5923/j.nn.20160601.01

Synthesis of Zinc Oxide / Polystyrene Nanocoposite Films and Study of Antibacterial Activity against Escherichia Coli and Staphylococcus Aures
Quraish A. Kadhim1, Riyadh M. Alwan2, Rawaa A. Ali2, Alwan N. Jassim2
1Chemical and Petrochemical Research Center, Corporation of Research and Industrial Development, Iraqi Ministry of Industry and Minerals, Iraq
2National Center for Packing and Packaging, Corporation of Research and Industrial Development, Iraqi Ministry of Industry and Minerals, Iraq
Correspondence to: Alwan N. Jassim, National Center for Packing and Packaging, Corporation of Research and Industrial Development, Iraqi Ministry of Industry and Minerals, Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study ZnO / Polystyrene nanocomposite thin films have been prepared via simple mixing route of polystyrene solution in toluene with ZnO solution. The films have been characterized by Atomic Force Microscopy (AFM) and Differential Scanning Calorimetry (DSC) analysis. Glass transition temperature (Tg), mid point, was found to be 82.45˚C for plain polystyrene film and increased to 89.26˚C for ZnO/Polystyrene nano composite film. AFM analysis indicated that Root Mean Square (RMS) is4.74 nm for plain polystyrene and found to be 6.2 nmfor ZnO /Polystyrene composite film. Results clearly showed that ZnO/Polystyrene nanocomposite films do have strong antibacterial activity against E. Coil and S. Aureus bacteria. These results suggest that the synthesized films based on ZnO nanoparticles have the potential as an active packaging material for food and pharmaceutical industries.
Keywords: Nano ZnO, Polystyrene film, Antibacterial activity, AFM
Cite this paper: Quraish A. Kadhim, Riyadh M. Alwan, Rawaa A. Ali, Alwan N. Jassim, Synthesis of Zinc Oxide / Polystyrene Nanocoposite Films and Study of Antibacterial Activity against Escherichia Coli and Staphylococcus Aures, Nanoscience and Nanotechnology, Vol. 6 No. 1, 2016, pp. 1-5. doi: 10.5923/j.nn.20160601.01.
Article Outline
1. Introduction
- The field of nanotechnology is one of the most popular areas of current research and development in basically all technical disciplines. This obviously includes polymer science and technology and even in this field the investigation coversa broad range of topics [1-5].Polymers are considered to be good hosting matrices for composite materials because they can easily be tailored to yield a variety of bulk physical properties and have a good processability [6-9]. Polystyrene, an amorphous, optically clear thermoplastic material which is flexible as thin film can be used as a host matrix because of its ideal properties for investigating the antimicrobial properties of the ZnO/PS nano composite.Inorganic nanoparticles possess outstanding optical, catalytic, magnetic and antimicrobial properties, which are significantly different from their bulk states [10-18]. Nanocomposites are as multiphase materials, where one of the phases has nano scale additives.One of the better-known materials that have been used for medical applications is zinc oxide nanoparticles. It is not too far from the truth to say that the ZnO is a magic material because of its wide area of applications. Reflecting the basic properties of ZnO, fine particles of the oxide have antibacterial and deoderizinc action, and for that reason are added into various materials including polymers, cotton fabric, rubber and food packaging [19-23]. Besides, ZnO nanoparticles are largely considerd due to their non toxic, biocompatible, cheap and easy to synthesis [24-28].Antimicrobials in food packaging are used to enhance quality and safety by reducing surface contamination of processed food, they are not a substitute for good sanitation particles [8]. Antimicrobials reduce the growth rate and maximum population of micro organism by extending the lag phase of microbes or inactivating them [29-34].Anyhow, nanoscale levels of metal oxides such as zinc oxide and magnesium oxide are being explored as antimicrobial materials for use in food packaging [6, 7, 9, 12].ZnO nanoparticles can be prepared easily by wet chemical method or via sol-gel, thermal decomposition and chemical vapor decomposition routes [35-40]. Besides, many authors proved the antimicrobial activity of nano ZnO [41-49].The present work focuses on the preparation and characterization of ZnO/polystyrene nano composite films. Besides, investigation of antibacterial activity of these films was conducted against E.Coli and S. Aureus bacteria.
2. Experimental Part
- All chemicals were purchased from Merck company. The solution was prepared by using distilled water. Preparation of nanozinc oxide illustrated else where [50]. In brief, gel of zinc oxide was prepared as follows:12.6g of zinc acetate dihydrate was dissolved in 400ml of distilled water, then 600ml of ethanol was added slowly at 50°C, and 6ml of H2O2 (47%) was added dropwise then mixed to get clear solution.To prepare ZnO/polystyrene nano composite film, in a typical experiment, 2g of polystyrene (Sabic company) was dissolved in 50ml of toluene and then directly added into the prepared gel of ZnO. The concentration of ZnO was taken as5 wt% ofto polystyrene. The solution mixtures were then poured into 10x15 cm glass mold. The solvents were evaporated slowly in a dust free chamber at room temperature, then composite films were obtained after evaporation and then heated for several hours at 80°C to remove any solvents and to convert zinc gel into zinc oxide nano particles. Also, for comparison a neat polystyrene film without nano material was prepared similarly to this procedure.
3. Antibacterial Activity
- The antibacterial activity of ZnO/Ps nanocomposite films were conducted against two kinds of bacteria. E. coli and S. aureus bacteria were obtained from the culture collection of food contamination center, ministry of science and technology (Baghdad-Iraq). And were re-confirmed before use.The stains were propagated on Trypical Soy Agar (TSA) at 37C for 18hrs. And maintaine at 0 to 2°C until use. Fresh cultivated bacterial colonies were suspended in 5ml of 0.85 normal saline. Turbidimetric measurements were used to determine the number cells in liquid media, and found to be 1.5x108 cell/ml based on standard 0.5 Mcfarland solution.The agar diffusion test was especially used for film samples [51]. The inoculum of E.coli and S. aureus bacteria was spread carefully on the surface of Muller-Hinton disk respectively and kept at room temperature for one hour. ZnO/Ps films were cut with surgical scissor into 1cm2 pieces. Each film sample was placed on the surface – inoculated Muller-Hinton disk for each kind of used bacteria and inoculated at 37°C for 18hrs. Plain polystyrene film was usedas control. Inhibition of bacterial growth on film specimens was used to determine the antibacterial activity in each case.
4. Results and Discussion
- The surface characteristic of plain polystyrene and ZnO/polystyrene nanocomposite films is determined by atomic force microscopy (AFM) as shown in figure 1, Root Mean Square (RMS) roughness of the films could be obtained from AFM analysis. The Roat Mean Squre Found to be 4.74 nm on the plain polystyrene surface, it was difficult to estimate the roughness of theinr surfaces while the value was 6.2 nm on the surface of ZnO/polystyrene nanocomposite film with 5 wt.% nano zinc oxide. Also, from AFM images we found the parlicles size of nano ZnO ranges from 60 to 80 nm. AFM measurements were carried out on A.A 300 scanning prope microscope from Angstrom Advanced Inc.
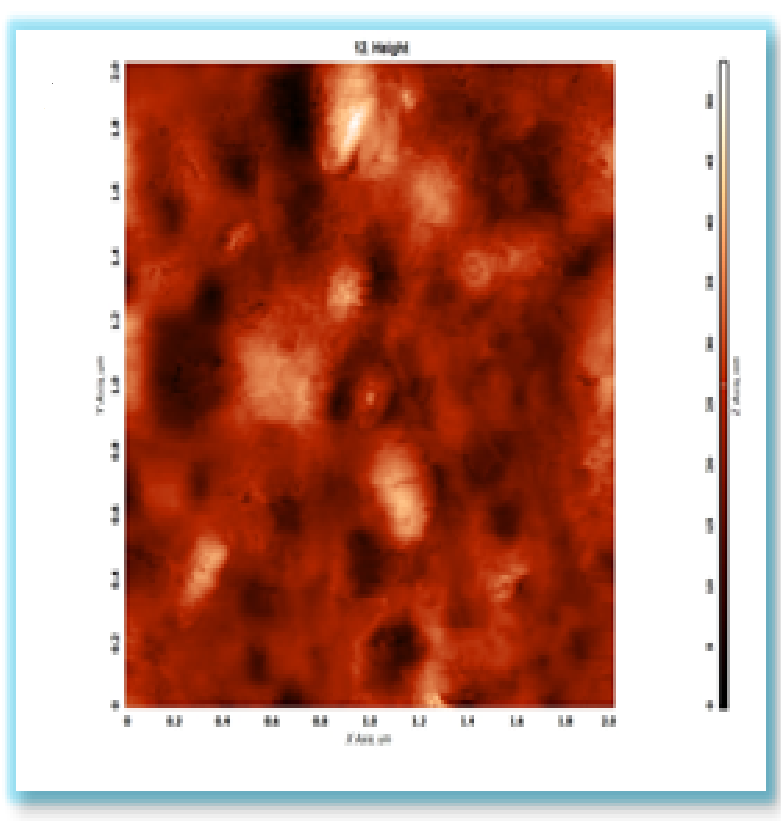 | Figure 1. AFM image of plain ZnO/polystyrene nanocomposite film with 5 wt% ZnO nanoparticles |
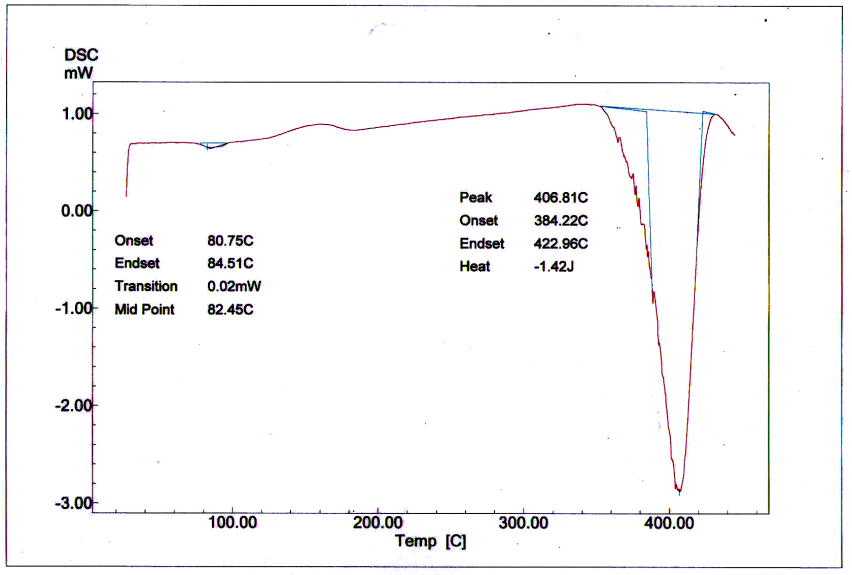 | Figure 2. DSC curve of plain polystyrene film |
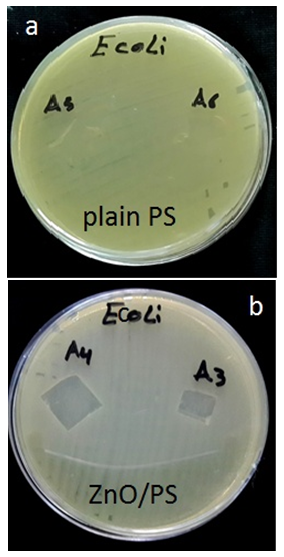 | Figure 3. Photo images showing antimicrobial activity on (a) plain polystyrene film, (b) ZnO/polystyrene nanocomposite film against E.coli by Disk diffusion method |
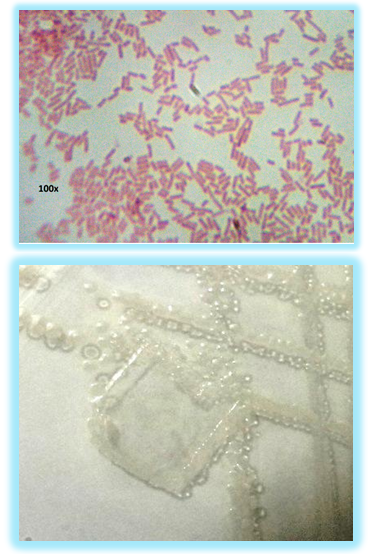 | Figure 4. Microscopic images show growth of E. Coli (ATCC:25922) bacteria on plain polystyrene film at different magnification (the test repeated three times for each sample) |
5. Conclusions
- We have demonstrated the synthesis of ZnO/polystyrene nanocomposite films with 5 wt.% of ZnO through the mixing process. The nano zinc oxide improved the thermal properties of the prepared composite films and AFM analysis showed the nano size of ZnO particales in polystyrene matrix. Also, the films displayed an excellent antibacterial activity against E.Coli and S. Aureus bacteria. The simplicity of the process means it would be easy to be scaled up to meet industry need. Finally, the originality in this work resides in the method for preparation the clear gel used in this experiment.
Funding / Support
- The authors wish to extend their gratitude to the Iraq ministry of industry and minerals for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML