-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(3): 64-69
doi:10.5923/j.nn.20150503.03
Preparation of Chitosan-Silver Nanoparticles in Nonaqueous Medium under Heating
Md. Ibrahim H. Mondal1, A. B. M. Nazmul Islam1, Md. Jahangir Alam2, Md. Mofakkharul Islam1, Md. Khademul Islam1
1Polymer and Textile Research Lab., Department of Applied Chemistry and Chemical Engineering, University of Rajshahi, Rajshahi, Bangladesh
2Department of Agronomy and Agriculture Extension, University of Rajshahi, Rajshahi, Bangladesh
Correspondence to: Md. Ibrahim H. Mondal, Polymer and Textile Research Lab., Department of Applied Chemistry and Chemical Engineering, University of Rajshahi, Rajshahi, Bangladesh.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Chitosan-silver nanoparticles were prepared in nonaqueous medium. In this work, sodium dodecyl sulfate was introduced into the dimethyl formamide solution during silver reduction from solution of its precursor salt AgNO3, acting as a stabilizing agent to prevent aggregation of silver nanoparticles, while chitosan was used as the solid support to embedded silver particles therein, resulting in chitosan-silver (CS-Ag) nanoparticle as suspension in the medium. The reaction started as homogeneous system which turned into heterogeneous with the formation of particles. The properties of CS-Ag nanoparticles were studied under two different salt concentrations and characterized by Atomic force microscopy, Scanning electron microscopy, Fourier transform infrared spectroscopy and Ultraviolet-visible spectroscopy (UV-Vis). Wide particle size distribution of synthesized nanoparticles depicts that concentration of AgNO3, which is responsible for the morphology, stability and particle size distribution, should be optimized, suggesting a lower salt concentration is favorable.
Keywords: Chitosan, Chitosan-Silver nanoparticles, Sodium dodecyl sulfate, Grain-rotation induced grain coalescence
Cite this paper: Md. Ibrahim H. Mondal, A. B. M. Nazmul Islam, Md. Jahangir Alam, Md. Mofakkharul Islam, Md. Khademul Islam, Preparation of Chitosan-Silver Nanoparticles in Nonaqueous Medium under Heating, Nanoscience and Nanotechnology, Vol. 5 No. 3, 2015, pp. 64-69. doi: 10.5923/j.nn.20150503.03.
Article Outline
1. Introduction
- There has been a tremendous increase in the number of patients affected by diseases like diabetes and cancer during the last decade. So, it has become the major focus of researchers to synthesize a compound that will be useful for the monitoring of certain elements that are present in excess quantities in the blood while used in the form of biosensors, at the same time being useful for the treatment of diseases like cancer. Chitosan (CS) is a polysaccharide composed of glucosamine and N-acetyl glucosamine linked with a β, 1→4 glycosidic linkage [1]. Chitosan is a biopolymer which is biocompatible and can be degraded by enzymes in human body, the degradation products are nontoxic. Commercial chitosans are semi-crystalline polymers and crystallinity plays an important role in adsorption efficiency [2]. Chitosan is a polymer which exhibits a broad-spectrum of antimicrobial activity by binding to the negatively charged bacterial cell wall followed by attachment to the DNA, inhibiting its replication [3, 4]. For the improvement of bioactivity on chitosan, it is often combined to other bioactive materials, such as drugs.Silver (Ag) nanoparticles have high therapeutic potential and exhibit good antimicrobial activity. Ag-nanoparticles have a wide range of antimicrobial activities and exhibit high performance even at a very low concentration. Ag-nanoparticles have been identified to possess good potential for the treatment of cancer [5]. But the major disadvantage of using silver alone is that it is not specific at targeting the cancer cells and also it is toxic to the normal cells when exposed for a longer time at the size of silver used is >20 nm [6]. CS–Ag nano composite is one of the rare composite materials that is seen to possess a capability of being used as a biosensor as well as in the treatment of cancer as the chitosan present in the nanocomposite is very specific to the cancer cells. It prolongs the action of silver on the affected cells while preventing the normal cell from the effect of silver. One more advantage of this nanocomposite is that it is biodegradable i.e., it can be degraded by the enzymes present in the body making it suitable for the treatment of cancer. Apart from the treatment of cancer, the nanocomposite also possesses good antimicrobial and biosensing activity [7].Sodium dodecyl sulfate (SDS) is introduced into the amide solution at the start of reaction or continuously during metal reduction [8]. SDS plays a twofold role it acts as a stabilizing agent to prevent aggregation of metal nanoparticles. In addition, SDS can be adsorbed on the surface of specific crystal faces and serve as shape control agent. In this work, we proposed the synthesis method by reducing AgNO3 in dimethyl formamide with chitosan by varying concentration for the preparation of silver nanoparticles. The influence of the salt concentration on the optical properties, structures and morphologies of CS-Ag nanoparticles was characterized by the Atomic force microscopy (AFM), Ultraviolet-visible spectroscopy (UV-Vis), Scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR).
2. Materials and Methods
2.1. Materials
- Prawn was collected from Rupsa ferry ghat, Rupsa, Khulna, Bangladesh in Figure 1. Prawn shell was separated from prawn for chitin. Sodium hydroxide pellet, hydrochloric acid and absolute alcohol were obtained from Merck, India; acetone was obtained from Merck, Germany; potassium bromide was obtained from Sigma ultra, USA; DMF from Fisher scientific, UK; SDS from Fluka analytical, Switzerland and silver nitrate form Uni-Chem, China.
 | Figure 1. Prawn shell |
2.2. Methods
2.2.1. Extraction of Chitin from Prawn Shell
- Prawn shell was washed with hot water and then dried in an oven at 105 °C for 72 h. Dried prawn shell was grounded. Deproteinization was carried out with NaOH (1 mol/L) at boiling temperature for 4 min in the prawn shell to NaOH solution ratio of 1: 16 w/v, followed by neutralization. Residue was demineralized with HCl (1 mol/L) at boiling temperature for 4 h in the material to HCl ratio of 1: 13 w/v. Excess acid was neutralized.
2.2.2. Conversion of Chitin into Chitosan
- Deacetylation of chitin was carried out using 40% NaOH in the Chitin to NaOH solution of 1: 20 (w/w) at boiling temperature for 3.5 h. After deacetylation solid was separated from alkali and extensively washed with distilled water to remove traces of alkali. Resultant solid was dried in a vacuum oven at 60°C for 24 h. The material obtained in this step is known as chitosan. Chitosan was grounded and stored in a desiccator [9].
2.2.3. Preparation of Chitosan-Silver Nanoparticles
- 0.05g of chitosan was dissolved in 20 mL of HCl (10 mole/L) solution and used as stock solution. 5 mL stock solution, 5 mL DMF and 0.05g of SDS were mixed for 30 min using a magnetic stirrer to get homogeneous solution. 4.90 mM silver nitrate salt was added in 10 mL DMF and mixed for 30 min using magnetic stirrer to get homogeneous solution. The total volume of solution was 20 mL and final concentration of AgNO3 was 2.45 mM, and these was placed in a three neck round bottom flask. Out of three neck of the round bottom flask, one neck was kept for solution upload, the other neck was used for thermometer and the middle one was used for condenser setup. When the effect of concentration of Ag precursor salts was examined, the concentration of AgNO3 was changed from 2.45 mM to 5 mM for the preparation of CS-Ag nanoparticles. The Ag/SDS molar ratio was kept at the same values as those used at initial condition. The solution temperature was increased from room temperature to 120°C by heating for 10 min.
2.2.4. Analyses of Size and Component of Products
- For AFM (Park Systems, XE-70, South Korea) and SEM (Hitachi, model-S 3400 N VPSEM, Japan) observations, CS-Ag particles were obtained from ethanol solution by centrifuging the colloidal solution at 15,000 rpm for 30 min in three times. Fourier transform infrared spectroscopy (Parkin Elmer, Spectrum 100, USA) and ultraviolet-visible (Shimadzu, UV-1650 PC, Japan) spectroscopy were also measured.
3. Results and Discussion
3.1. AFM Observation
- Freshly prepared nanoparticles were centrifuged with ethanol, followed by casting onto glass slide for film and subsequent dried in open air at room temperature. Figure 2 depicts AFM images of CS-Ag nanoparticles with different metal ion concentrations such as 2.45 mM and 5 mM.In this study we used AFM to investigate the morphological changes and structural damage to CS–Ag nanoparticles under various condition such as salt concentration, heating time. The AC mode was used to acquire both the topographical and phase AFM images during the scan (Figures 2 and 3). The phase image provides the degree of phase shift of the cantilever oscillation relative to the signal sent to the base of the piezo driving the cantilever. By recording the phase shift of the cantilever oscillation, the phase imaging goes beyond simple topographical mapping to detect variations in chemical composition, adhesion and other surface properties. The topographical and the corresponding phase images Figures 2(a1-c2) and 3(a3-b4) show the typical near spherical shaped form. In contrast, a dramatic change of the CS-Ag composite can be seen after treatment with silver salt concentration. The morphologies of CS-Ag nanocomposite were dependent on several factors including polymer solubility, solvent evaporation, total thickness, molecular weight and surface composition [10]. Furthermore, the corresponding phase images or grain boundaries (Figures 3(a3-b4)) are affected by the local topography and highlight variations in the tip sample interaction which are consistent with important modification in the surface properties due to concentration fluctuation from 2.5 mM to 5 mM of AgNO3 salt concentration at constant parameters.
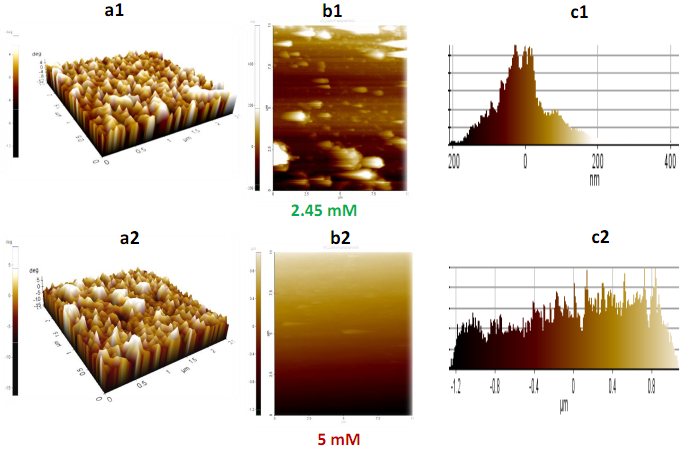 | Figure 2. 2(a1-c1) and 2(a2-c2) represent topographical height and frequency histogram of CS-Ag nanoparticles using 2.45 mM and 5 mM of AgNO3 salt respectively |
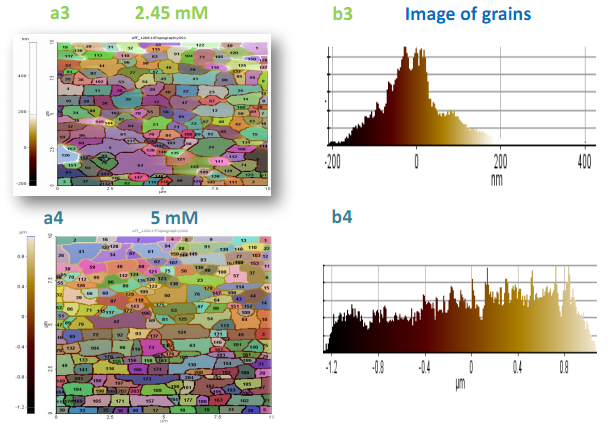 | Figure 3. 3(a3 - b3) and 3(a4 - b4) reveal the grain size of nanoparticles obtain from 2.45 mM and 5 mM of AgNO3 concentration respectively |
3.2. Effect of Precursor Salt Concentration
- The effect of precursor salt concentrations is examined by changing silver nitrate concentrations from 2.45 mM to 5 mM to prepare CS-Ag particles. The molar ratio of Ag salt and SDS was kept constant for the preparation of CS-Ag particles in reaction medium.Figures 2(a1-c2) show AFM data at 2.45 mM, and 5 mM of AgNO3 salt solution after heating for 10 min. In AFM topography, shown in Figures 2(a1-c1), topographical images and histogram frequency depict that the size distribution of the most prepared CS-Ag nanoparticles are in the range of 1-200 nm using 2.45 mM salt and major products are spherical or plate like CS-Ag particles. The dissolved silver ion is reduced to small (CS-Ag) particles that are abundantly dispersed in solution shown in Figures 3(a3-b3). At 5 mM CS-Ag nanocomposites size distribution are in the range of 1-800 nm, no significant shape change occur in CS-Ag particles but major products are larger and nearly spherical and/or bar like CS-Ag particles produced that are bigger than 2.45 mM salt concentration in Figures 3(a4-b4). These results imply that shape, and size distributions of the product are strongly depend on the metal ion concentration and lower AgNO3 (2.45 mM) concentration is favorable for the preparation of CS-Ag particles with nearly spherical and/or plate like shape. It predicted that the increasing of salt concentration change in phase angle due to differing amount of damping experienced by the probe tip as it rusters across the sample surface increases, indicating formation of coarser particles.
3.3. SEM Observation
- The surface morphology of synthesized CS–Ag nanocomposite was analyzed using SEM technique. The SEM image of CS depicted that the particles are in the form of bundles with a leaf morphology in Figure 4a. The SEM images of CS-Ag nanoparticles depicted that the shape of particles are nearly spherical or plate like shaped in Figure 4b for 2.45 mM. Figure 4b shows a mixture of CS and Ag wherein Ag nanoparticles are seen to be enveloped by the chitosan (CS) polymer indicated in Figure 4b [11]. In case of 5 mM concentration, the synthesized particles are in the form of aggregates or larger in size than that of 2.45 mM CS-Ag particles in Figure 4c. It is observed that Ag nanoparticles are embedded in a matrix of chitosan. The morphology of CS-Ag nanoparticles was predominantly nearly spherical or plate like in Figure 3c and aggregated into larger irregular structure with no well-defined morphology observed in the micrograph of Figure 4d under heating or storage.
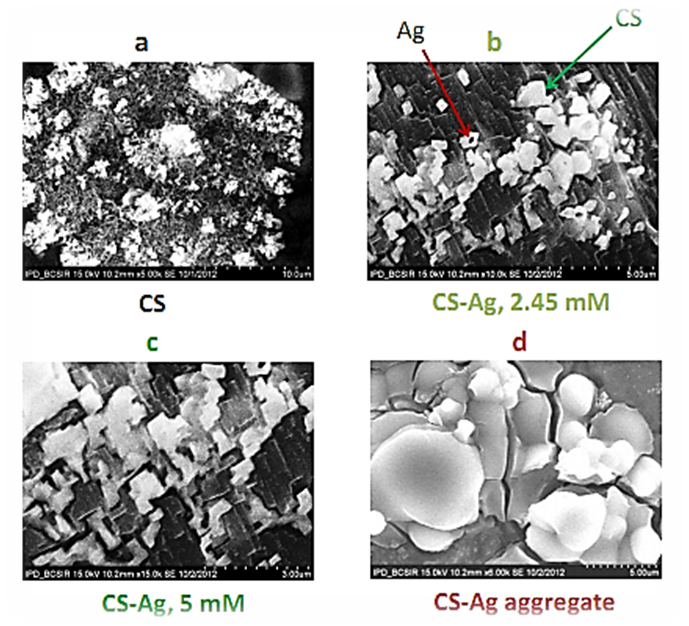 | Figure 4. a, b, c and d represent SEM micrograph of freshly prepared chitosan, CS-Ag nanoparticles for 2.45 mM, 5 mM and aggregated nanoparticles under heating respectively |
3.4. FTIR Observation
- FTIR observation is measured by using infrared spectrophotometer (Perkin Elmer, Spectrum 100, USA). KBr diluted nanoparticles are washed with alcohol and centrifuged to collect particles, subjected to give the FTIR spectra of chitosan and CS-Ag nanoparticles are depicted in Figure 5. The band at 3411 and 3467 cm-1 correspond to O-H stretching H-bonded alcohols and phenols of CS-Ag nanoparticles and chitosan itself respectively. The peak at 1667 and 1622 cm-1 correspond to N-H bond primary amines of CS-Ag nanoparticles and chitosan itself respectively. The peak at 2835 and 2831 cm-1 correspond to O-H stretch carboxylic acids of CS-Ag nanoparticles and chitosan itself respectively. The peak at 1402 and 1383 cm-1 correspond to C-N stretching of aromatic amine group of CS-Ag nanoparticles and chitosan itself respectively. The other bands are of C–O stretch alcohols and carboxylic acids observed at characteristics spectral shifts. herefore the synthesized CS-Ag nanoparticles are surrounded by chitosan having functional groups of amines, carboxylic acids, alcohols and esters.
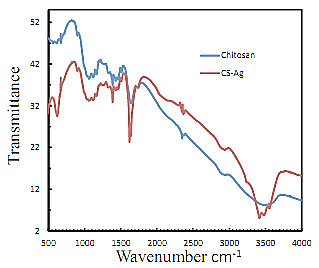 | Figure 5. FTIR spectra of silver nanoparticles stabilized in chitosan and pure chitosan |
3.5. UV–Visible Spectroscopy Analysis
- The UV-Visible spectral measurements were carried out using UV-Visible spectroscopy. UV–Visible absorption spectra recorded is quite sensitive to the formation of Ag nanoparticles because of the fact that Ag nanoparticles exhibit an intense absorption peak due to surface plasmon resonance (SPR). Figure 6 depicted the UV–Vis spectra of Ag nanoparticles prepared from two different concentrations of AgNO3 (2.45 mM, and 5 mM) with chitosan (5 mL) for 10 min heating. All spectra exhibit an absorption band in the range of 300–400 nm with a typical plasmon resonance band of CS-Ag nanoparticles. The absorption against wavelength curves at various concentrations is shown in Figure 6. It was noticed that the reduction capacity of chitosan increased with varying concentration of Ag salt. As the salt concentration increases, possibly more and more of hydroxyl groups are converted to carbonyl groups by air oxidation, which in turn reduces more Ag+ at a constant chitosan concentration. A single strong peak with a maximum around 295 nm was observed in the UV–Vis spectra, which corresponds to typical SPR of conducting electrons from the surface of silver nanoparticles. The intensity of the absorption of solutions increased with increase in the concentration of AgNO3 up to 5 mM at constant heating time 10 min. Ag nanoparticles with nearly spherical morphology are embedded in the chitosan matrix which arise a small peak at around 380 nm in Figure 6. As the particles increase in size, the absorption peak usually shifts toward the red wavelength [12].
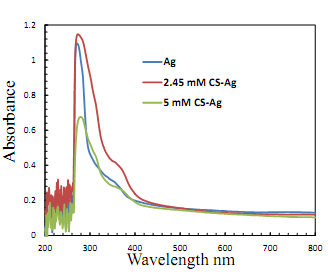 | Figure 6. UV-visible spectra of pure Ag salt solution, CS-Ag nanocomposite obtained from 2.45 mM and 5 mM of AgNO3 concentrations |
3.6. Mechanism
- The growth mechanisms of CS-Ag nanostructures under different preparation routes are summarized in Figure 7. In this process a large amount of CS-Ag nanoparticles are prepared. Mechanism in Figure 7 is the grain-rotation induced grain coalescence (GRIGC) and fusion mechanism [13]. According to this model, the rotation of grains among neighboring grains results in a coherent grain grain interface which leads to the coalescence of neighboring grains via the elimination of common grain boundaries, thus forming a larger grain. In our case, single crystal, particles having well defined faces were lost after the formation of large polycrystalline spherical particles. Thus, during GRIGC process fusion of polygonal crystals leading to polycrystalline spherical or plate like particles. In the Ostwald-ripening mechanism, the atoms from one particle undergo dissolution and then they are transferred to another particle. There is a net atomic transport from the particles with sizes smaller than the average value to larger particles. Particles smaller than the average value will shrink or even disappear.
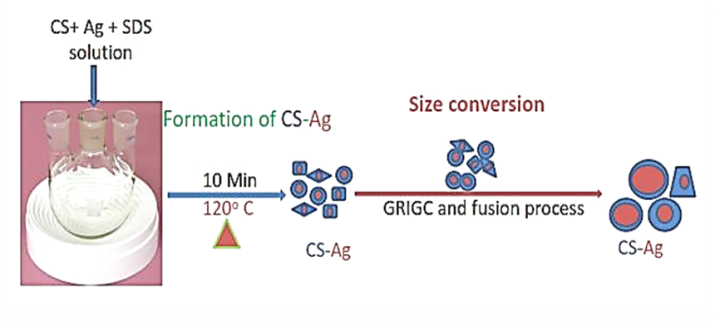 | Figure 7. Schematic diagram of CS-Ag nanocomposite formation and their shape and size conversion at 120 °C heating for 10 min |
ACKNOWLEDGMENTS
- The authors would like to acknowledge the Ministry of Education in Bangladesh for funding the project as Higher Education Research Grant in 2014 (Memo no.: 37.01.0000.078.02.018.13-206(38)/6-35, 2014).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML