-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(3): 57-63
doi:10.5923/j.nn.20150503.02
Morphology and Elemental Study of Silicon Nanoparticles Produced Using a Vibratory Disc Mill
E. A. Odo
Department of Physics, Federal University Oye-Ekiti, Oye-Ekiti, Nigeria
Correspondence to: E. A. Odo, Department of Physics, Federal University Oye-Ekiti, Oye-Ekiti, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The morphology and elemental composition of silicon nanoparticles, produced by means of a vibratory disc mill in a top-down synthesis route has been investigated, using scanning electron microscopy (SEM), energy dispersive x-ray spectroscopy (EDX) and transmission electron microscopy (TEM). Both SEM and TEM reveals that the particles consisted of a wide range of size distributions, ranging from a few faceted particles about 1-3 μm in size, to a large number of much rounder particles in the 100nm range. This size distribution pattern fits in well with a log-normal distribution function, with a mean size being observed to shift to the lower particle size ranges with increasing milling time. The EDX analysis reveals that the level of contaminants, which may have resulted from reactions with the milling medium, as well as with the atmospheric oxygen, remains insignificant for the duration of the milling process.
Keywords: Nanoparticles, Microscopy, Morphology
Cite this paper: E. A. Odo, Morphology and Elemental Study of Silicon Nanoparticles Produced Using a Vibratory Disc Mill, Nanoscience and Nanotechnology, Vol. 5 No. 3, 2015, pp. 57-63. doi: 10.5923/j.nn.20150503.02.
1. Introduction
- Nanostructured materials have received much interest and intense research efforts in recent times because of their great potential for engineering and scientific applications [1]. Annexing such geometry scaling, and other novel properties of semiconductors at this length scale will lead to great improvement in electronic device performance [2]. The ability to produce silicon nanoparticles in large quantities, has accelerated the development of printed electronic devices on flexible substrates [3, 4]. One of the stark limitation to the application of bulk nanostructured materials, however, is their poor ability to provide fully dense and defect-free parts [1]. The route that is generally employed to circumvent this shortcoming in the production of isolated and uncontaminated nanostructured particles, is to use either inert gas condensation [5], or chemical reactions of a precursors. In the case of silicon, this has traditionally involved one of two forms of thermal processing: pyrolysis of silanes [6-9], or evaporation of bulk silicon in an inert atmosphere [10, 11]. Of these, two methods, the most widely used is the decomposition of silane gas, which can be achieved by using simple thermal pyrolysis [5] or laser decomposition [8], to produce particles with sizes in the range of 100nm. However, the application of soft chemical synthesis methods to create silicon nanoparticles, is limited by their complexity, as it involves multi-stage synthesis [12] or growth in inverse micelle structures, as well as by their low yield [13].The high cost and complexity of the above mentioned route limits its application, predominantly to the laboratory [14]. The “top-down” approaches, employing high energy mechanical milling or chemical reduction, which are widely used in the industrial production of metal and ceramic powders, have been successfully employed for silicon and other notable semiconductor materials too [15]. Mechanical milling in particular enables the low-cost production of nanostructured materials with the added advantage being simplicity and effectiveness. Many reports have however suggested, that mechanical milling of crystalline materials often results in particles that vary in size and structure, varying from isolated spherical particles to agglomerate of complex structures. Therefore, a major concern associated with this method is the control of the resulting physical features, such as the average diameter and morphology of the particles. Another concern is the possible contamination of the processed materials by the milling medium [16, 17].In this work, standard techniques SEM and TEM was used to investigate the propagation of morphological properties (size and shape) of silicon nanoparticles produced through mechanical attrition of silicon wafer by a vibrator disc mill. The elemental compositions of the produced particles, and the proliferation of the contaminants resulting from the milling process was also studied as a function of milling time.
2. Experimental Procedure
- Batch milling of two types of bulk silicon was done by using a Siebtechnik 800W TS laboratory vibratory disc mill, equipped with a 52100 chrome steel pestle and mortar [18]. The bulk silicon sources used in this work were, p-type silicon wafers and, for comparison purposes, a metallurgical grade silicon (more specifically, a 2503 grade polycrystalline silicon metal), provided by Silicon Smelters (PTY) Ltd. Polokwane, South Africa. The purity of the p-type silicon wafer was nominally 99.99%, while the metallurgical grade silicon, according to the manufacturer’s analysis, contained 99.40% silicon. Table 1 lists the known elemental impurities constituting the remaining 0.60% of the metallurgical grade silicon. The milling process pulverized the materials by means of impact and friction, usually in a few minutes, while at the same time homogenizing the grounded powder. The first set of powder produced came from milling the silicon wafers for 1 hour through to 5 hours. The second set came from milling the metallurgical grade silicon for 3 hours.
|
3. Results and Discussion
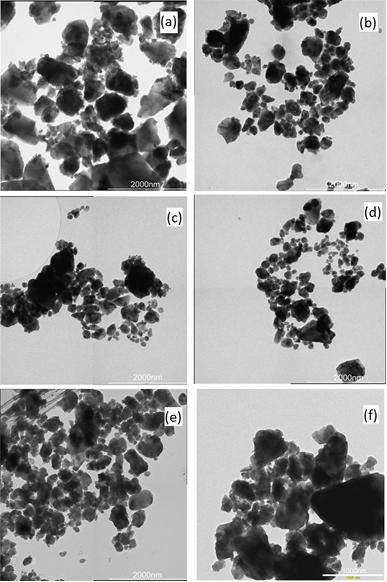
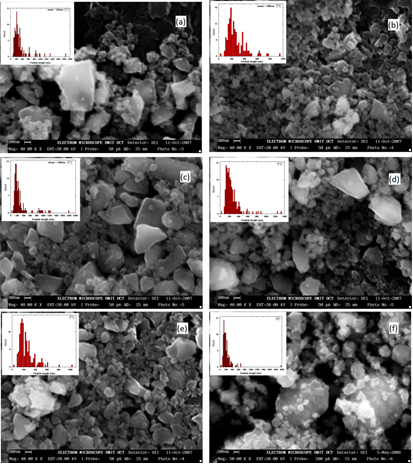
- Fig. 1 (a) - (e) are micrographs of the powder milled for 1-5 hours. The morphology and size distribution of the particles in the powder after an hour of milling as seen in Fig. 1 (a), shows that the powder consisted of particles with a wide range of sizes and two predominant shape type. On the one hand are large faceted particles, cleaved along closely packed planes, with sizes ranging from 1 – 3μm, and on the other hand are relatively smaller particles, with sizes ranging from 100 – 400nm with a generally spherical shape. The size distribution, based on a random measurement of 150 particles that have clearly defined boundaries along their longest axis, shows a typical log-normal distribution pattern. The more spherical particles (100 – 400nm) were found to dominate the distribution.After 2 hours of milling, the resulting powder exhibited a similarly wide size distribution pattern like that after 1 hour of milling. Comparing the two powder, the proportion of the particles in the size range of 100 – 400nm was observed to have increased after 2 hours. The average particle size was also observed to have shifted toward the lower numbers. Further milling of the powder up to 5 hours, (as seen in Fig. 1 (c) - (e)), shows a progressive increase in the proportion of the particles towards the size range of 100 – 400nm resulting from a consistent reduction of the larger faceted particles with milling time. Also worth mentioning is the presence of clustering of the smaller particles into super clusters of relative larger sizes. Fig. 1 (f) is a micrograph of the metallurgical grade silicon after it has been milled for 3 hours. It has a similar size distribution as the 3 hours milled powder produced from the wafer. Unlike the milled wafer powder that has large faceted particles in the size range 1 – 3μm, the larger particles, in this case are predominately spherical with smoother surfaces.
 is clearly the dominant element in the powder constituting about
is clearly the dominant element in the powder constituting about 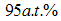 , with oxygen at
, with oxygen at 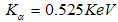 , iron at
, iron at 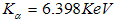 and chromium at
and chromium at 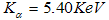 constituting only trace amounts in the powder. No clear peaks were found in the different energy positions indicated by the arrows, corresponding to the energy levels for the possible contaminants. The trace amount of contaminant detected within the instruments resolution is indicative that the contamination from the milling medium and the possible atmospheric oxidation from the milling process were insignificant.
constituting only trace amounts in the powder. No clear peaks were found in the different energy positions indicated by the arrows, corresponding to the energy levels for the possible contaminants. The trace amount of contaminant detected within the instruments resolution is indicative that the contamination from the milling medium and the possible atmospheric oxidation from the milling process were insignificant. 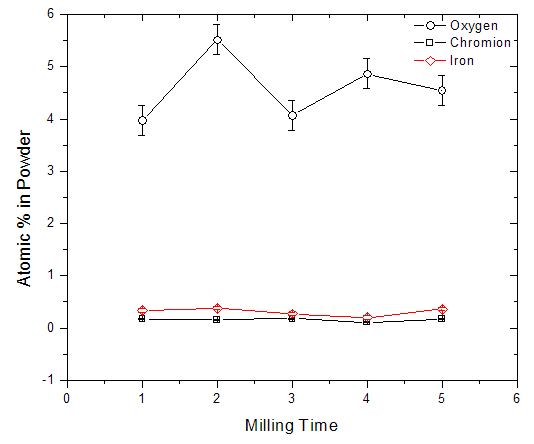 | Figure 3. Showing the levels in atomic % of detected oxygen, chromium and iron in the milled powder remaining relatively constant for the entire milling period |
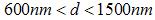 . There are also scattered clusters of particles, consisting of the relatively smaller particles in the range of
. There are also scattered clusters of particles, consisting of the relatively smaller particles in the range of 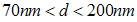 , where
, where 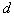 represents the length of the longest cross-section of the particle, with the smallest measured particle recorded, been
represents the length of the longest cross-section of the particle, with the smallest measured particle recorded, been 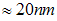 .
.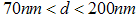 becomes dominant.A closer look at a larger grain at higher magnification (Fig. 5) shows darker areas either overlaying or embedded in the particle (the areas indicated in the dotted circle). This may be the result of either the agglomeration and compacting of small particles into relatively bigger clusters due to the hammering process in the mill, or the result of plastic deformation of particle, thus giving rise to stacks of crystallites, with planes of orientation different to those of the initial material [19].
becomes dominant.A closer look at a larger grain at higher magnification (Fig. 5) shows darker areas either overlaying or embedded in the particle (the areas indicated in the dotted circle). This may be the result of either the agglomeration and compacting of small particles into relatively bigger clusters due to the hammering process in the mill, or the result of plastic deformation of particle, thus giving rise to stacks of crystallites, with planes of orientation different to those of the initial material [19].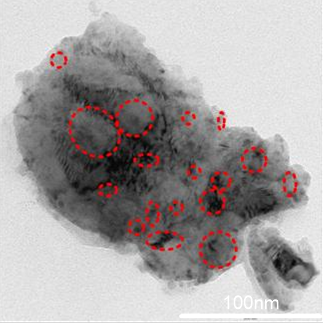 | Figure 5. TEM micrograph of a single large grain showing either an agglomeration of smaller particles or a possible stacking of re-oriented crystallites |
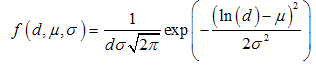 | (1) |
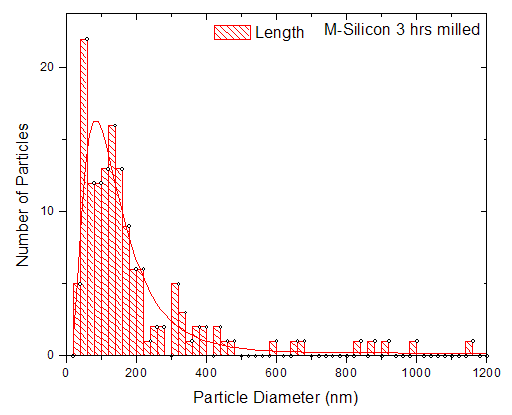
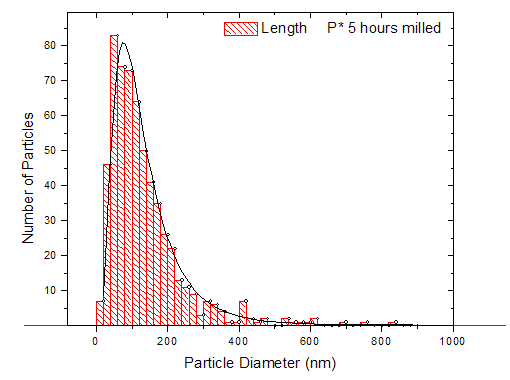 | Figure 6. Particle size distribution for powder 5 hours milled silicon wafer fits well to a log-normal pattern with a mean cross-section size of 113nm |
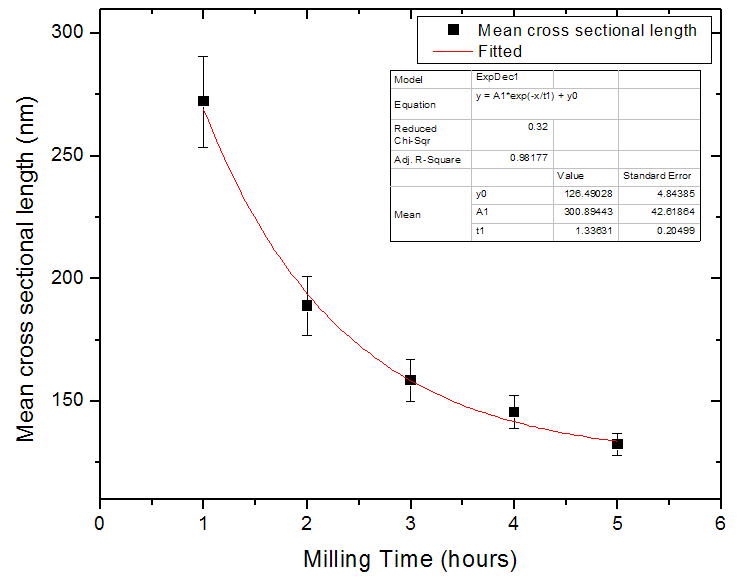 | Figure 8. The variation of the mean cross-sectional particle sizes for the milled wafer with increasing milling time |
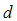 is the cross-sectional length of the particle,
is the cross-sectional length of the particle, 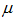 and
and 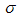 are the logarithmic mean and standard deviation respectively. The fit of this function to the size distribution histogram was used to derive a mean particle size for each of the milled powders. The center position of the fitted function, which corresponds to the average cross-sectional length, was plotted against milling time in Fig. 8. The plot shows a decrease in the average particle size with milling time, and this data can be well represented with a first order exponential decay function given as,
are the logarithmic mean and standard deviation respectively. The fit of this function to the size distribution histogram was used to derive a mean particle size for each of the milled powders. The center position of the fitted function, which corresponds to the average cross-sectional length, was plotted against milling time in Fig. 8. The plot shows a decrease in the average particle size with milling time, and this data can be well represented with a first order exponential decay function given as,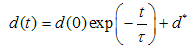 | (2) |
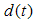 describes the particle diameter after milling for a time
describes the particle diameter after milling for a time 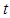 ,
, 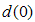 is the initial particle diameter,
is the initial particle diameter, 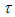 defines a time constant and
defines a time constant and 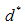 represents a constant size value for the particles diameter after a very long milling time.A fit of (2) to the data of Fig. 8 yields a value for
represents a constant size value for the particles diameter after a very long milling time.A fit of (2) to the data of Fig. 8 yields a value for 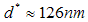 . It is reasonable to conclude that this value represents the average cross-sectional size of particle that can be achieved after a long time of milling using this method. A similar analysis carried out on the metallurgical silicon powder can be adequately represented by a log-normal distribution function, centered at 96nm.From the above, it is reasonable to suggest that the production of silicon powder from a single crystalline silicon wafer, using a vibratory disc mill in a top-down synthesis route, starts with cleavage of the wafer along its preferred orientation, followed by a gradual attrition of the particles surfaces toward the smaller particle size, reaching a fairly constant size distribution after about 3 hours of milling. The effect of further milling only reduces more of the larger particles to the size range 100 nm – 400nm.The shape and size distribution, as observed for the five hours milling of the wafer is comparable to those obtained from high-energy ball milling of single crystalline silicon for 50 hours reported by Di-Guerra et. al [19]. This finding suggests that the present method is a fast and efficient method of producing silicon nanoparticles from its bulk state.While cleavage and later attrition is true for the wafers, this may not be the case for the metallurgical silicon. The differences in the surface texture of particles derived from the wafers, which have sharp edges, and those derived from the metallurgical silicon bulk, which have smoother round surfaces, suggests that the process of reduction in the metallurgical powder is due to attrition alone. However, the size distribution after 3 hours of milling is comparable in both systems.
. It is reasonable to conclude that this value represents the average cross-sectional size of particle that can be achieved after a long time of milling using this method. A similar analysis carried out on the metallurgical silicon powder can be adequately represented by a log-normal distribution function, centered at 96nm.From the above, it is reasonable to suggest that the production of silicon powder from a single crystalline silicon wafer, using a vibratory disc mill in a top-down synthesis route, starts with cleavage of the wafer along its preferred orientation, followed by a gradual attrition of the particles surfaces toward the smaller particle size, reaching a fairly constant size distribution after about 3 hours of milling. The effect of further milling only reduces more of the larger particles to the size range 100 nm – 400nm.The shape and size distribution, as observed for the five hours milling of the wafer is comparable to those obtained from high-energy ball milling of single crystalline silicon for 50 hours reported by Di-Guerra et. al [19]. This finding suggests that the present method is a fast and efficient method of producing silicon nanoparticles from its bulk state.While cleavage and later attrition is true for the wafers, this may not be the case for the metallurgical silicon. The differences in the surface texture of particles derived from the wafers, which have sharp edges, and those derived from the metallurgical silicon bulk, which have smoother round surfaces, suggests that the process of reduction in the metallurgical powder is due to attrition alone. However, the size distribution after 3 hours of milling is comparable in both systems.4. Conclusions
- In this study silicon nanoparticles were produced via a top-down synthesis route, from both single crystalline and a polycrystalline bulk form, using a laboratory disc mill. The milled silicon nanoparticles powders produced via this process showed a continuous reduction of particle size with milling time. In the case of powder from the single crystalline bulk (i.e. the wafers), the process started with cleavage along its main crystal orientation, followed by attrition of the edges of the particles as they became smaller. In the case of powders from the polycrystalline bulk, the reduction process appeared to be due to attrition alone. In general, the produced powders irrespective of the starting bulk materials were composed of an agglomeration of smaller particles, resulting in bigger clusters. The effect of milling time on the contaminant level was negligible. The detected oxygen level remained fairly constant for the entire milling period, suggesting minimal silicon oxidation from the process, and the measured value was most likely an intrinsic oxygen level that was already part of the bulk.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML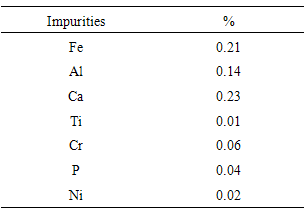
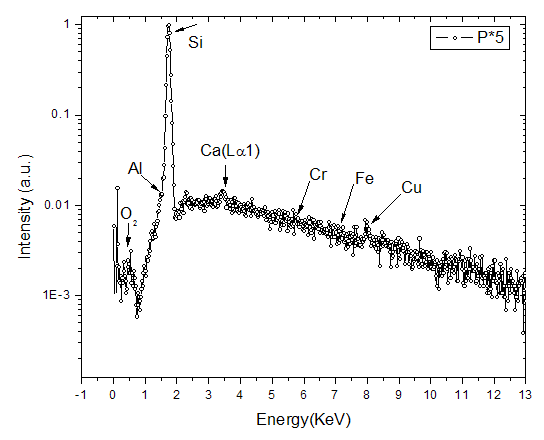
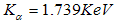 as the dominant element, with possible contaminants like oxygen, chromium and iron appearing in trace amount
as the dominant element, with possible contaminants like oxygen, chromium and iron appearing in trace amount