-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(2): 36-44
doi:10.5923/j.nn.20150502.03
Nanoscale Electrochemical Polishing and Preconditioning of Biometallic Nickel-Titanium Alloys
Tarek M. Abdel-Fattah1, Jon Derek Loftis1, Anil Mahapatro2
1Applied Research Center, Jefferson National Laboratory, Newport News and Department of Molecular Biology and Chemistry, Christopher Newport University, Newport News, USA
2Department of Biomedical Engineering, Wichita State University, Wichita, KS, USA
Correspondence to: Tarek M. Abdel-Fattah, Applied Research Center, Jefferson National Laboratory, Newport News and Department of Molecular Biology and Chemistry, Christopher Newport University, Newport News, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Nickel-Titanium is a medical-grade alloy used extensively in medical implants and devices in the biomedical field. Nickel-Titanium was successfully electropolished via an ecologically friendly and biocompatible ionic liquid medium based on Vitamin B4 and resulted in nanosized surface roughness and topography. Voltammetry and chronoamperometry tests determined optimum polishing conditions for the Nickel-Titanium alloy while atomic force microscopy and scanning electron microscopy provided surface morphology comparisons to benchmark success of each electropolishing condition. Energy dispersive X-ray analysis combined with scanning electron microscopy resulted in significantly smoother Nickel-Titanium surfaces for alloy samples tested while indicating that the constituent metals comprising each specimen effectively electropolished at uniform rates.
Keywords: Electrochemical polishing, Biocompatible treatment, Vitamin B4, Ni-Ti Alloy, Biomedical Alloys
Cite this paper: Tarek M. Abdel-Fattah, Jon Derek Loftis, Anil Mahapatro, Nanoscale Electrochemical Polishing and Preconditioning of Biometallic Nickel-Titanium Alloys, Nanoscience and Nanotechnology, Vol. 5 No. 2, 2015, pp. 36-44. doi: 10.5923/j.nn.20150502.03.
Article Outline
1. Introduction
- Metal alloys are comprised of two or more of different metals, and these alloys incorporate the physical and chemical properties of all constituent metals in the process of being smelted. Common reasons for combining metals include, modification of melting point, improving thermal or electrical conductivity, increased structural integrity, along with potential benefits of improved tensile and shear strength [1, 2]. Metal alloys have been shown to possess numerous relevant applications in science, especially in the biomedical industry, ranging from prosthetic artificial limb replacements to coronary artery stents and are commonly referred to as biometallic alloys [3, 4]. Examples of prominent biometallic alloys include stainless steel alloys [5], cobalt-chromium alloys [6, 7], and titanium alloys [8-14].A medical-grade alloy, Nickel-Titanium (Ni-Ti), is the focus of this research endeavor, and has been recently used extensively in medical devices and in the biomedical field as a result of its ferroelastic capacity as an effective shape memory alloy. When in contact with blood, biometallic alloys including Ni-Ti, may trigger a variety of iatrogenic reactions, including surface-mediated thrombosis, complement activation and device-centered infection [15, 16]. A significant body of research clearly demonstrates that in-vivo tissue compatibility may be modified by varying surface chemistry and topography as these changes guide alterations in the morphology and the orientation of adhering cells, thus enhancing biocompatibility [16]. Therefore, a variety of surface treatments are commonly performed on medical devices prior to use or implantation to promote and enhance corrosion resistance and biocompatibility [17, 18]. For many years, electrochemical polishing (electropolishing) has been used to smooth conductive surfaces and to perform surface passivation on biomaterials [19-25]. Electropolishing of metals provide long lasting benefits [26-29] for individuals who have undergone surgeries and have received an implant containing biomedical alloys. Some of the benefits of electropolishing include prolonged lifetime of implants and reduced metal release into the bloodstream, both of which protect the longevity of the surgical implant [26-29].Reports of electropolishing of Ni-Ti usually cite use of acids such as sulfuric acid, nitric acid, phosphoric acid, and other acid-based formulations as the electrolyte in the electrochemical cell [6, 30]. Any residual acid present on the implant could have a detrimental effect on implant-cell interactions. Escalating awareness of these complications in the practical utilization of biometallic alloys in various applications including coronary stents or prosthetic limb replacement, has perpetuated the need for non-acid electrolytes to be used during the electrochemical polishing of metal implants [31, 32]. Thus, electrochemical studies outdating the previous processes of electropolishing via controlled acid corrosion have been gaining significance in recent years [19-25]. In this paper, we report the electrochemical polishing of Ni-Ti in a non-acid based electrolyte to produce nanoscale controlled surfaces. The electropolishing efficiencies of Ni-Ti with a non-acid ionic liquid-based deep eutectic solvent based on vitamin-B4 and ethylene glycol will be demonstrated. Surface characterization will be conducted prior to and after electropolishing treatments to survey the surface roughness and elemental concentrations correlated with electropolishing parameters such as applied current density, voltage, and duration of polishing treatments.
2. Experimental Methods
- Electrochemical polishing tests were conducted on 5 identical Ni-Ti samples, each with a polishing area of ½ cm2. Electropolishing consisted of acid-free treatments using an ionic liquid prepared from vitamin-B4 (VB4, choline chloride, Acros Organics 99%), and ethylene glycol (EG, Sigma-Aldrich 99.8%); both chemicals were used as-received. The ionic liquid mixture was prepared by stirring the two components together at a molar ratio of 2 ethylene glycol to 1 vitamin-B4 (2EG:1VB4) at 70°C until a homogeneous colorless liquid was formed.Electropolishing was carried out in a standard electrochemical cell with a three electrode system and the ionic liquid (2EG:1VB4) was prepared as the electrochemical polishing medium. Electropolishing procedures consisted of a platinum counter-electrode plate accompanied with a silver wire reference and the Ni-Ti alloy as the working electrode. Electrodes were degreased using deionized water and acetone to preserve the purity of samples during testing. The working electrode was abraded with 150-grit glass paper, rinsed, and dried prior to recording each measurement to ensure reproducible voltammetric results. All trials were performed at 70°C with chronoamperometric tests set to 900 s. Linear sweep voltammetry was utilized to step voltage from 0-8 V to determine an ideal current range for each sample to conduct electrochemical polishing. Chronoamperometry was used to fix the voltage for electrochemistry experimentation; while the charge was optimized to limit undesired variability. Linear sweep voltammetry and chronoamperometry experiments were conducted using a Gamry PCI4-G750 potentiostat and controlled using the accompanying framework and e-chem Analyst (v.5.5) software. Voltammetric experiments were performed at a constant scan rate of 20 mV/s. After electropolishing treatments, surface characterizations were made using Atomic Force Microscopy (AFM), Scanning Electron Microscopy (SEM), and Energy Dispersive X-Ray Spectroscopy (EDX). The surface topography and roughness were analyzed using AFM; or specifically, a Dimension 3100 Digital Instruments manufactured Nanoscope IV Scanning Probe Microscope with software version 6.12 in resonant (tapping) mode. Tapping mode was utilized to determine roughness analyses for the metal alloy sample both prior to and post electropolishing treatments. This study reports root mean square calculations for all roughness average characterizations, such that standard deviation is accounted for in the average statistic provided. In supplemental AFM imagery provided for each section, root mean square data (listed as RMS Rq data in AFM frames) was selected over roughness average data (listed as Ra; below RMS Rq in AFM frames) for its capacity to best summarize the conditions present at the sample surface being characterized. SEM and EDX provided additional characterization for the alloy samples. These tests provided details of surface morphology via SEM and percent composition analyses through EDX before and after electropolishing treatments of Ni-Ti specimens. The imaging mode used to facilitate sweeps from 0-10 keV allowed for detection of all elements included in the EDX library (the library contains all periodic table elements). All SEM imagery in this study was recorded at 25 kV with listed magnification settings and representative scale. EDX was utilized to survey and report % composition of the alloy surveyed to supplement scanning electron microscope (SEM) imagery recorded before and after each treatment via a JEOL JSM 6060 LV equipped with WINEDS High Performance X-Ray Micro-Analysis software.
3. Results and Discussion
- The Ni-Ti sample surfaces were characteristic of effective electrochemical pretreatment (ECPT), and were successfully electropolished using a non-acid ionic liquid (2EG:1VB4). Visual observations of surface-treated sample areas presented a shiny surface with increased optical reflectivity and a smoother appearance and feel as compared to untreated sample area (Figure 1).
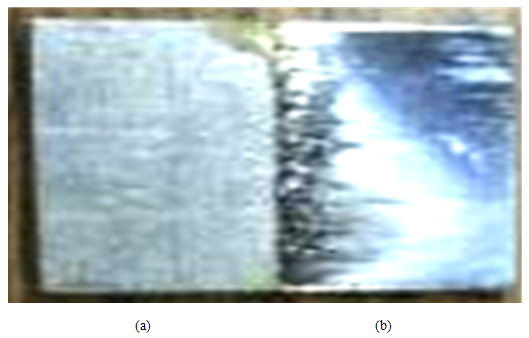 | Figure 1. Photograph of Ni-Ti sample depicting separate regions (a) before electropolishing and (b) after electropolishing |
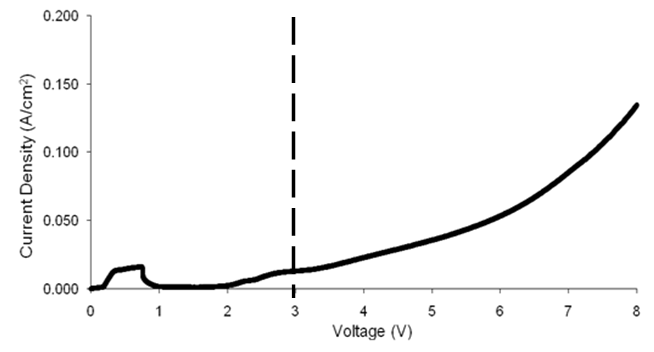 | Figure 2. Linear sweep voltammetry scan for a Ni-Ti sample at a scan rate of 20 mV/s. The dashed line indicates the ideal voltage (3 V) utilized for chronoamperometry experiment |
|
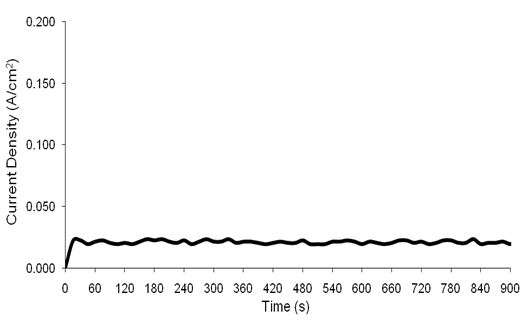 | Figure 3. Chronoamperometry scan (15 min) for a Ni-Ti sample carried out at 3 V and 70ºC with the current ranging from 0-0.147 A/cm2 |
 | Figure 4. AFM images of a Ni-Ti sample in 2D (top) and 3D (bottom) prior to electropolishing treatments |
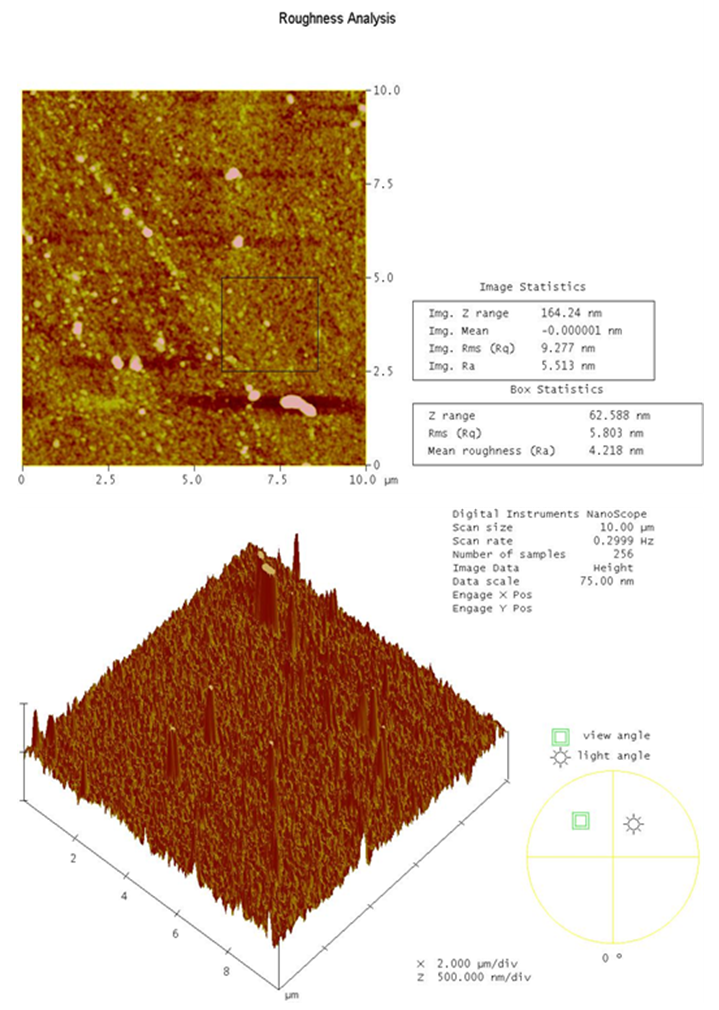 | Figure 5. AFM images of a Ni-Ti sample in 2D (top) and 3D (bottom) after electropolishing treatments |
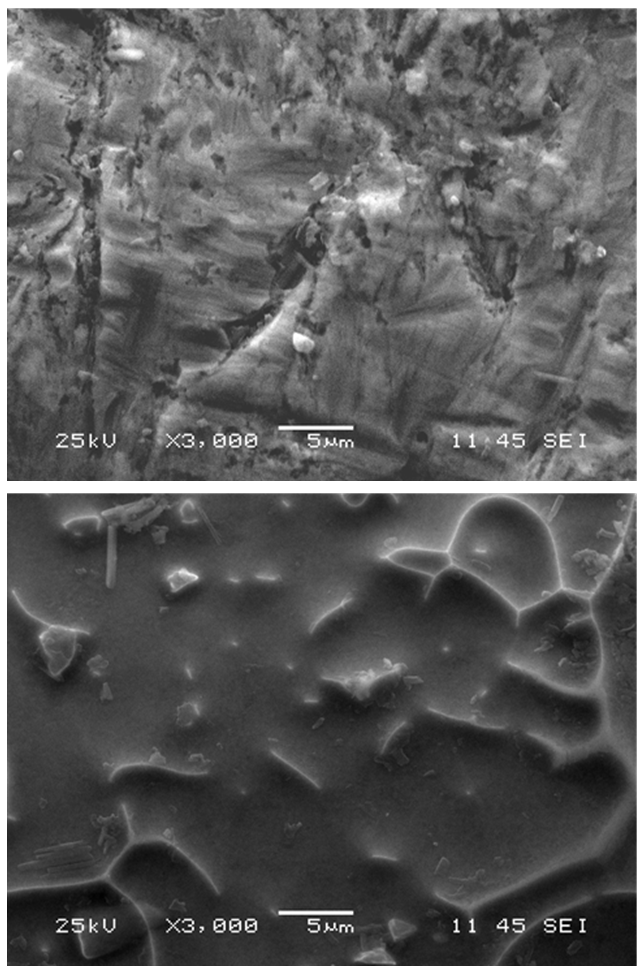 | Figure 6. SEM image Ni-Ti alloy before (top) and after (bottom) electropolishing with the ionic liquid |
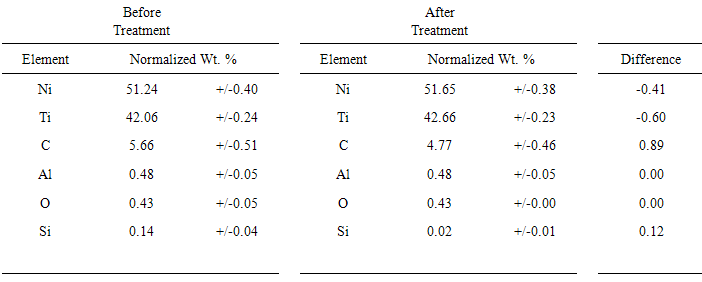
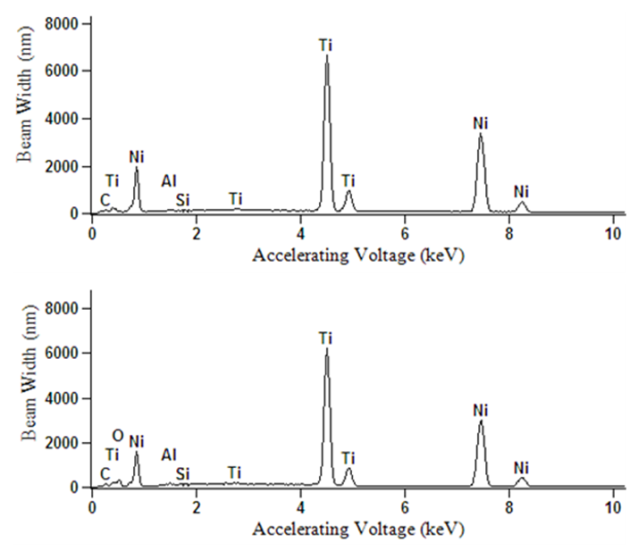 | Figure 7. EDX data for Ni-Ti prior to (top) and post-electropolishing (bottom) |
4. Conclusions
- In this study, we demonstrated an ECPT of medical-grade Ni-Ti surfaces using 2EG:1VB4 as an electropolishing medium. Surface roughness of the ECPT of Ni-Ti using the ionic liquid resulted in surface roughness of the specimen being significantly reduced from 64.4 nm to 11.4 nm without significantly altering the alloy elemental composition. This study provides an electrochemical polishing pretreatment method to effectively control the relative smoothness of Ni-Ti implants at the nanoscale (less than 20 nm) which could offer many potential biomedical applications including controlling cell interactions at biointerfaces.
ACKNOWLEDGMENTS
- The authors would like to gratefully acknowledge the financial support from NSF award number: 0959807.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML