-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(2): 30-35
doi:10.5923/j.nn.20150502.02
Synthesis, Characterization and Optical Properties of L-Arginine Stabilized Gold Nanocolloids
A. L. Sunatkari1, S. S. Talwatkar2, Y. S. Tamgadge3, G. G. Muley4
1Department of Physics, Siddhartha College of Arts, Science and Commerce, Fort, Mumbai, India
2Department of Physics, N.G. Aacharya and D.K. Maratha College of Arts, Science and Commerce, Chembur, Mumbai, India
3Department of Physics, Mahatma Phule Arts, Commerce & S.R.C. Science College, Warud, India
4Department of Physics, Sant Gadge Baba Amravati University, Amravati, India
Correspondence to: G. G. Muley, Department of Physics, Sant Gadge Baba Amravati University, Amravati, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
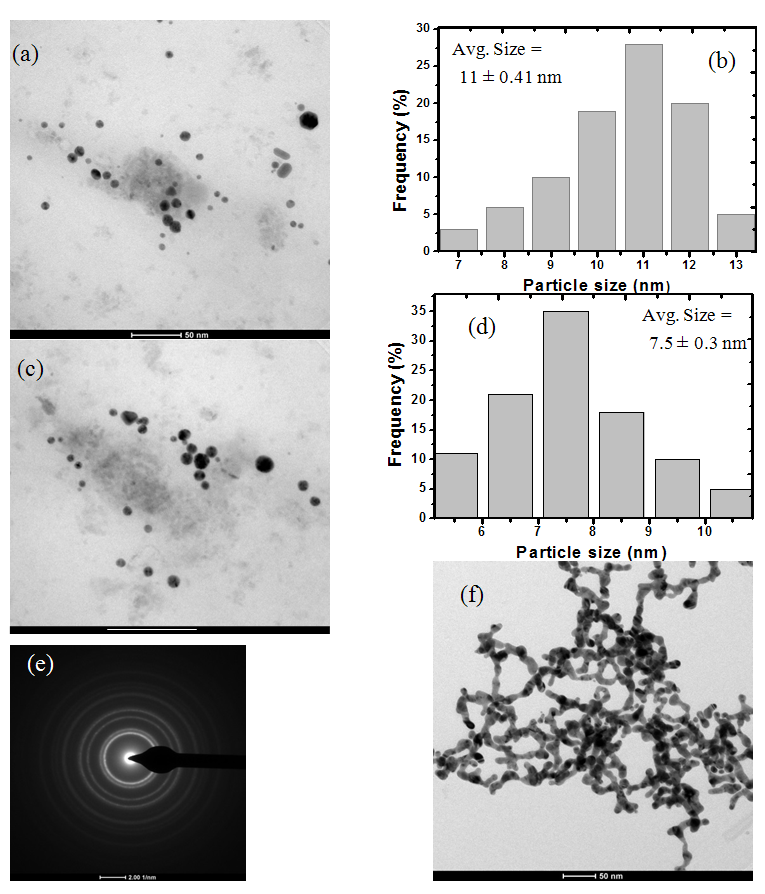
We investigate the effect of surface passivation using L- arginine on Localised surface plasmon resonance (LSPR) and size of colloidal Gold Nanoparticles (AuNPs) synthesized by chemical reduction method. Highly stable colloidal gold nanoparticles (AuNPs) stabilised in L-Arginine were synthesized. Optical absorption spectra of colloidal AuNPs exhibit an absorption peak at 520 nm due to Surface Plasmon Resonance. As the L-arginine concentration increased from 1 mM to 5 mM, blue shift in the LSPR peak position noticed. Fourier transform infrared spectra show the shift in absorption band position due to donor-acceptor interactions between L-arginine functional groups and AuNPs surface suggesting strong encapsulation of AuNPs. The size of the AuNPs is also found to be reduced from 11 to 7.5 nm with the stabilizer concentration as analysed using transmission electron microscopy. The X-ray diffraction study reveals face-centred cubic (fcc) structure of AuNPs.
Keywords: L-arginine capping, UV-Visible, FTIR, TEM, Gold nanoparticles
Cite this paper: A. L. Sunatkari, S. S. Talwatkar, Y. S. Tamgadge, G. G. Muley, Synthesis, Characterization and Optical Properties of L-Arginine Stabilized Gold Nanocolloids, Nanoscience and Nanotechnology, Vol. 5 No. 2, 2015, pp. 30-35. doi: 10.5923/j.nn.20150502.02.
Article Outline
1. Introduction
- Metal nanoparticles (MNPs) are an important class of nanomaterial due to their size-dependant properties and potential applications in sensors, catalysis and data storage [1-2]. Generally, Because of their high surface energy, MNPs are unstable and need to be stabilized against aggregation by suitable surface modifying agents. The common choice of functional groups for AuNPs surface modification are cyano (-CN), mercapto(-SH), thiol-mediated binding of ligands are known to have high affinity for gold and expected to produce quite small sized AuNPs [3]. Numerous reports are available on the effect of surface passivation on the properties of MNPs, which would play a vital role in the applications such as biosensors [4], DNA/drug delivery [5], Imaging [6], bio diagnostic and optoelectronics devices [7]. Common choice for MNPs surface modification is thiol-mediated binding of ligands [8-9]. Aniline, long-chain amines, carboxylic compounds have been used as stabilizers in the synthesis of MNPs [10]. Researchers have also studied the role of Poly-Vinyl Pyrrolidone (PVP), polyacrylate and polyacrylamide as protective agents which can effectively alters shape, size, stability and linear optical properties of AgNPs [11]. More recently, researchers have diverted attention to the binding of metal NPs with amino-acids [12]. Amino acids are inherently compatible, and one of the common amino acid is L-arginine, which has zwiterrionic structure. Upon functionalization of MNPs with L-arginine molecules, they can highly facilitate the interaction and hence have a potential to bring drastic changes in optical properties. Joshi et. al. reported the synthesis of L-Lysine capped gold nanoparticles [13]. Bhargava et.al reported the synthesis of gold nanoparticles using the amino acids L-tyrosine, glycyl-L-tyrosine, and L-arginine [14]. To the best of our knowledge, reports are not available on the use of L-arginine as protective agent in the synthesis of AuNPs. Therefore, we have used L-arginine for surface modification in the synthesis of AuNPs.Various methods such as sol-gel method, laser ablation, and chemical reduction have been used to synthesize gold nanoparticles. Among these, chemical reduction method [15-18] is extensively used because of its simplicity and accuracy. The objective of this work is to prepare AuNPs in colloidal form with various concentration of L-arginine reduced by sodium Borohydride by wet chemical (reduction) method and to discuss the effect of L-arginine concentration on LSPR, size of AuNPs, and stability of gold colloids.
2. Experimental Section
2.1. Chemicals
- Gold (III) chloride hydrate (HAuCl4, 99.999 % purity), sodium borohydride (NaBH4, 98% purity), L-arginine (99% purity) and PVP (MW 10000) purchased from Sigma-Aldrich and used without further purification. De-ionised water was used in synthesis process. L-arginine was used as capping agent and NaBH4 was used as a reducing agent.
2.2. Synthesis of L-arginine Stabilised AuNPs Colloid
- Stock solutions of 5 mM HAuCl4, 10 mM NaBH4 were prepared. L-arginine solutions with 1, 2.5, and 5 mM concentrations were prepared by dissolving appropriate amount in double distilled water. All solutions were kept in ice-bath for 20 min. In 200 ml volumetric flask, 30 ml double distilled water, 10 ml NaBH4 and 20 ml L-arginine solutions were taken and stirred at 50°C for 20 min. AuNPs colloidal solution was obtained by drop wise addition of 20 ml gold precursor into the above mixture. Solution was turned dark violet (stable colour) in colour in 10 min indicating formation of AuNPs. AuNPs were precipitated by centrifugation for 20 min. at 4000 rpm. Precipitate washed with methanol to remove free legends and then dried by means of optical heating at 40°C. As-prepared AuNPs colloids were stable for long duration of 3 months.
2.3. Characterizations
- Absorption spectra were recorded covering wavelength range 200-900 nm using ultraviolet-visible (UV-vis) spectrometer (Black-C-SR, Stellarnet Inc. USA). Average size and shape of nanoparticles were determined form transmission electron micrograph (TEM) recorded using transmission electron microscope (JEM 2100F, JEOL) with point to point resolution of 0.19 nm and accelerating voltage 200 kV. Structural study of nanoparticles was carried out with X-ray diffractometer (Miniplex-II, Rigaku) with CuKα wavelength 1.54 Å in the angle 2θ ranging from 20 to 80o. Fourier transform infrared (FT-IR) spectra were obtained with machine 3000 hyperion microscope with vertex 80 FTIR system (Bruker, Germany) in the range of wavelength 450 - 7500 cm-1 with resolution of 0.2 cm-1.
3. Results and Discussion
3.1. UV-vis Absorption Spectroscopy
- Optical absorption spectra of L-arginine capped gold nanocolloids reduced with sodium borohydride synthesized by chemical reduction method is recorded in the wavelength range of 200 nm - 900 nm at room temperature. Fig. 1 illustrates the results obtained for various concentration of L-arginine.
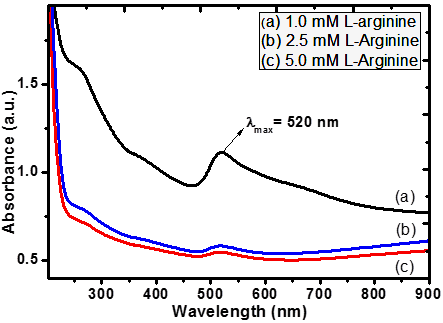 | Figure 1. Absorption spectra of AuNPs colloids with (a) 1, (b) 2.5 and (c) 5 mM L-arginine concentrations |
3.2. FT-IR Study
- Fig. 2 shows FT-IR spectra of L-arginine capped AuNPs taken in the wavelength range of 400 cm-1 - 4000 cm-1. Generally, in the synthesis of AuNPs surfactant ligands that binds to their surfaces which stabilize the nuclei and large nanoparticles against aggregation by electrostatic repulsive force. The electrostatic repulsive force is due to the equally charged L-arginine molecules adsorbed on the AuNPs surface through its electron-donating end group. Because of the electrostatic interaction between AuNPs surface (acceptor) and L-arginine ligands (donor), stable nanoparticles can be formed [25]. FT-IR is a powerful tool to investigate the bonding between surface of AuNPs and L-arginine molecule. Previously, L-arginine demonstrated a very good surfactant in the synthesis of CdS and ZnS nanoparticles by Talwatkar et.al. [26].
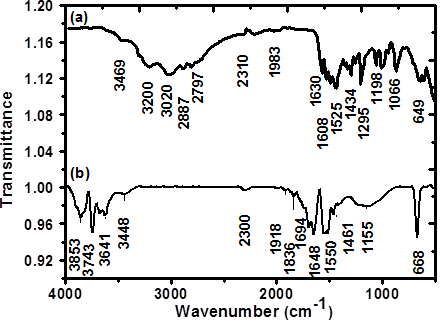 | Figure 2. FT-IR spectrum of (a) Pristine L-arginine and (b) AuNPs capped by L-arginine |
3.3. Structural Analysis
- X-ray diffraction (XRD) study gives the information about morphology and phase formation of L-arginine capped AuNPs. Fig. 3 shows XRD spectrum of AuNPs embedded in PVP polymer matrix recorded in the range of 20 to 80o for 2θ values.
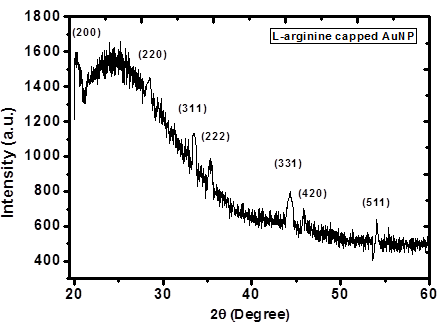 | Figure 3. XRD spectrum of 1 mM L-arginine stabilised Au-PVP thin film |
4. Conclusions
- In summary, highly stable AuNPs were obtained using L-arginine as a stabilizer. The effect of L-arginine concentration on LSPR, size of AuNPs was studied. The average size of AuNPs, as analysed by TEM, was found to be decreasing with increase in L-arginine concentration. The presence of fcc structured AuNPs was confirmed from XRD studies. The blue shift in the LSPR peak position is noticed. The strong bonding between AuNPs and L-arginine functional groups confirmed from FT-IR study. Results proved that L-arginine is a very good stabilizing agent.
ACKNOWLEDGEMENTS
- Author (A. L. Sunatkari) is grateful to the University Grants Commission (UGC), India for providing financial assistance under Minor Research Project (47/1690/10). Authors acknowledge the support of Director, SAIF facility, IIT-Bombay, Mumbai for providing FT-IR and TEM facilities and Chairman, DST-FIST, SGB Amravati University, Amravati for providing XRD facility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML