-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(2): 23-29
doi:10.5923/j.nn.20150502.01
Green Synthesis of Silver Nanoparticles: Synthesis, Characterization and Antibacterial Activity
Thanaa I. Shalaby1, Ola A. Mahmoud2, Gihan A. El Batouti3, Ebtihag E. Ibrahim1
1Department of Medical Biophysics, Medical Research Institute, Alexandria University, Alexandria, Egypt
2Department of Microbiology, Medical Research Institute, Alexandria University, Alexandria, Egypt
3Department of Microbiology and Immunology, Faculty of Pharmacy, Pharos University, Alexandria, Egypt
Correspondence to: Gihan A. El Batouti, Department of Microbiology and Immunology, Faculty of Pharmacy, Pharos University, Alexandria, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The synthesis of metal and semiconductor nanoparticles is an expanding research area due to the potential applications for the development of novel technologies. In this work, we describe a cost effective and environment friendly technique for green synthesis of silver nanoparticles and evaluate their antibacterial activity from silver nitrate solution through the extract of Zingiber officinale rhizome as a reducing as well as a capping agent. The growth of nanoparticles was monitored by UV-vis spectrophotometer and complemented with characterization using Transmission Electron Microscopy, X-ray Diffraction and Fourier Transform Infrared Spectroscopy. Transmission Electron Microscopy revealed the presence of mono-dispersed silver nanoparticles with an average size of 3.1 nm. X-ray diffraction studies corroborated that the biosynthesized nanoparticles were crystalline silver. Fourier Transform Infra-Red spectroscopy analysis showed that the synthesized nano- silver were capped with bimolecular compounds which were responsible for the reduction of silver ions. The antibacterial effect of these nanoparticles were studied against Staphylococcus aureus and Escherichia coli. This study indicated that silver nanoparticles posses considerable antibacterial activity in comparison with standard antibacterial agents, and hence further investigation or clinical applications is necessary.
Keywords: Antibacterial activity, Fourier Transform Infra-Red Spectroscopy, Green synthesis, Silver nanoparticles, X-Ray Diffraction, Zingiber officinale
Cite this paper: Thanaa I. Shalaby, Ola A. Mahmoud, Gihan A. El Batouti, Ebtihag E. Ibrahim, Green Synthesis of Silver Nanoparticles: Synthesis, Characterization and Antibacterial Activity, Nanoscience and Nanotechnology, Vol. 5 No. 2, 2015, pp. 23-29. doi: 10.5923/j.nn.20150502.01.
Article Outline
1. Introduction
- Nanotechnology can be termed as the synthesis, characterization, exploration and application of nano-sized (1-100nm) materials for the development of science. It deals with the materials whose structures exhibit significantly novel and improved physical, chemical, and biological properties. Nanotechnology holds a promising future for the design and development of many types of novel products that are used in early detection, treatment, and prevention of various diseases. [1]Synthesis and characterization of nanoparticles is an emerging field for research. Preparation of nanoparticles requires processes that include chemical and physical techniques. With the development of several chemical-synthetic techniques, the concern for environmental contamination is also heightened as the protocols used for chemical synthesis require some toxic chemicals for their synthesis. Most physical methods deal with enormous consumption of energy to maintain the high pressure and temperature employed in the synthesis procedures. To overcome these chemical and physical hazards, the adoption of green chemistry has been pursued to minimize bio-hazardous waste. [2] The use of environmentally friendly material as plant extracts, bacteria and fungi offers numerous benefits of eco-friendliness and compatibility for pharmaceutical and biomedical applications. [3, 4]Due to their exclusive properties, silver nanoparticles (Ag-NPs) may have several applications, including their use as catalysts, [5] as optical sensors, in spectrally selective coatings for absorption of solar energy, [6] in textile engineering, in electronics, [7] and most importantly in the medical field as a bactericidal and as a therapeutic agent. [8, 9] Ag-NPs have high reactivity due to the large surface to volume ratio and play a crucial role in inhibiting bacterial growth in aqueous and solid media. [10] The investigation of this phenomenon has regained importance due to the increase of bacterial resistance to antibiotics. Ag-NPs obtained by methods conveying green synthesis are candidates to be used in biological systems. [11].The aim of this work was to study the synthesis and characterization of Ag-NPs using Zingiber officinale rhizome extracts as a reducing and capping agent (green pathways). Their antibacterial activity was also evaluated.
2. Material and Methods
2.1. Preparation of the Reagent
- Silver nitrate (AgNO3) was purchased from Sigma Aldrich and fresh ginger rhizome (Zingiber officinale) was purchased from a local market. A 10 gm of thoroughly washed ginger rhizome was used to prepare the extract. The upper part was removed by a knife, chopped into the fine pieces and converted into a paste using mortar-pestle. This was followed by the addition of 40 ml deionized water, that was boiled for 2 minutes and filtered, then centrifuged at 5000 rpm for 15 minutes. The supernatant was collected to be further used as ginger rhizome broth for the experiment.
2.2. Synthesis of Ag-NPs
- A 50 ml of 1mM silver nitrate (AgNO3) was heated to boiling. To this solution, 10 ml of ginger rhizome broth was added for reduction of silver ions (Ag+), then kept at room temperature for one hour. The color transformation from yellow to yellowish brown indicated the formation of Ag-NPs. [12]
2.3. Characterization of Ag-NPs: [13, 14, 15]
- The color change in reaction mixture (metal ion solution and ginger extract) was recorded through visual observation. The bio-reduction of Ag+ in aqueous solution was monitored by measuring the spectrum of the solution within the range of 350–600 nm using UV-Vis-spectrophotometer (Unicam UV5-220), and a quartz cuvette with water as the reference. To study the stability of nanoparticles colloidal solution, the solution was kept at room temperature for one week. During this period UV-Vis spectrum of the solution was measured at different time intervals; after half hour, 24hrs and one week. The color and pH of the solution were also checked at regular intervals, which hardly showed any change. The size and morphology of the nanoparticles were examined using a transmission electron microscope (TEM, JEOL 100 CX). The sample was prepared by placing a drop of Ag-NPs on a carbon-coated copper grid and was then left to dry in air, before transferring it to the microscope. The crystalline nature of Ag-NPs was analyzed by X-ray diffractometer (Shimaduz, XRD- 7000, Maxima, Japan), operated at a voltage of 30 KV, a current of 30 mA, with CuKα radiation and analyzed between 5 and 100 (2 θ). The crystallite domain size was calculated from the width of the XRD peaks, assuming that they were free from non-uniform strains, using the Scherrer's equation as follows:
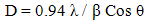 | (1) |
2.4. Antibacterial Assay
- The antimicrobial susceptibility of green synthesized Ag-NPs was evaluated using the disc diffusion method and growth inhibition test. Pure slant cultures of Staphylococcus aureus (S.aureus) and Escherichia coli (E.coli) were obtained from the Microbiology Department of the Medical Research Institute (MRI) Alexandria, Egypt. [16]
2.4.1. Disc Diffusion Method
- Bauer-Kirby’s disc diffusion method was used. Müller-Hinton Agar was prepared from a commercially available dehydrated medium (Oxoid®) according to manufacturer’s instructions. The dried surface of Mueller- Hinton agar plates were inoculated with two pathogenic bacterial strains; one with a Gram-positive bacteria, S.aureus and one with a Gram-negative bacteria, E. coli by swabbing over the entire sterile agar surface. Sterile paper discs made of Whatman filter paper (Np:1), 6 mm in diameter were loaded with Ag-NPs (50µL, 3mM).Two standard antibiotic discs; Gentamycin and Ciprofloxacin (Oxoid®), were also placed in each plate to be used as control antibiotics. The cultured agar plates were incubated at 37°C for 24 hours, after which the zones of inhibition were observed.
2.5. Estimation of Colony Forming Units
- E.coli was used for the estimation of colony forming units (CFU) on solid agar plates. Freshly grown bacterial inoculum of (105 cells/ml) of E.coli was inoculated into tubes containing 500 µl liquid Tryptone Soy Broth medium (T.S.B). They were left to grow in a shaking incubator at 37C, with 200 rpm for 3 hours. The tubes were then treated with different concentrations of Ag-NPs (0, 20, 40, 60 µg/ml). These samples were diluted at 106 folds to obtain better discrete colonies, and were thereafter spread on nutrient agar plates. After incubation at 37°C for 24 hours, the numbers of CFU were observed.
3. Results and Discussion
- In this study, ginger was as biological system for the synthesis of Ag-NPs. Oxalic acid, ascorbic acid, phenyl-propanoids and zingerone are present in the chemical composition of ginger [17]. Ascorbic acid and/or oxalic acid are/is the main organic components in the extract which play(s) the role of reducing Ag+ into Ag-NPs. The possible stages of the formation of Ag-NPs from ginger extract during the chemical reaction included nucleation, condensation, surface reduction and stabilization as illustrated in Figure 1.
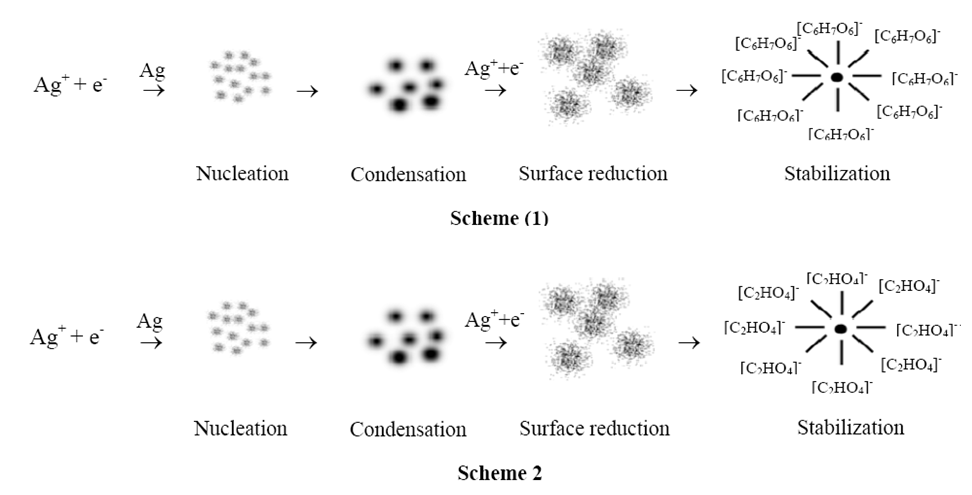 | Figure 1. Two schemes represent the possible stages of the formation of Ag-NPs from ginger extract |
3.1. Characterization of Ag-NPs
- The color transformation from yellow to yellowish brown indicates the formation of Ag-NPs [12]. These colors arise due to the excitation of surface Plasmon vibrations in the metal nanoparticles [15]. UV–vis spectroscopy is an indirect method to examine the bio-reduction of Ag-NPs from aqueous AgNO3 solution. It was found that, the green synthesized Ag-NPs were very stable in the solution, even one week after their synthesis, which validates the application of ginger (Zingiber officinale) rhizome as a biomaterial for the synthesis of nano-sized Ag particles. The UV-Vis absorption spectra of green synthesized Ag-NPs using ginger extract (Figure 2) showed that the surface Plasmon band occurred at around 430 nm indicating the presence of Ag-NPs. [14] It was observed that the peak was blue shifted in the absorption spectrum from 430 nm to 450 nm as a function of time. This indicated that the Ag-NPs solution was extremely stable for more than one week, with no signs of aggregation even at the end of this period.
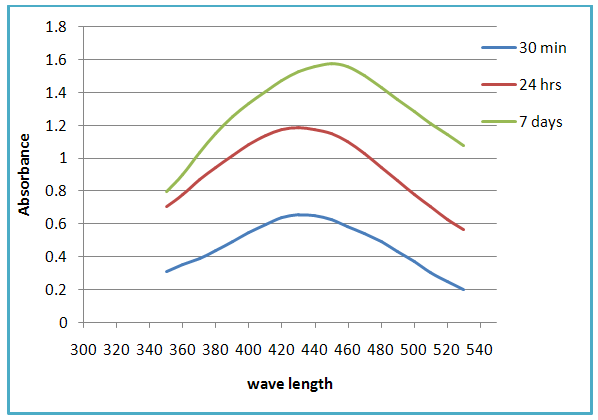 | Figure 2. UV-vis absorption spectra recorded as function of time for silver nanoparticles using rhizome extract of Zingiber officinale |
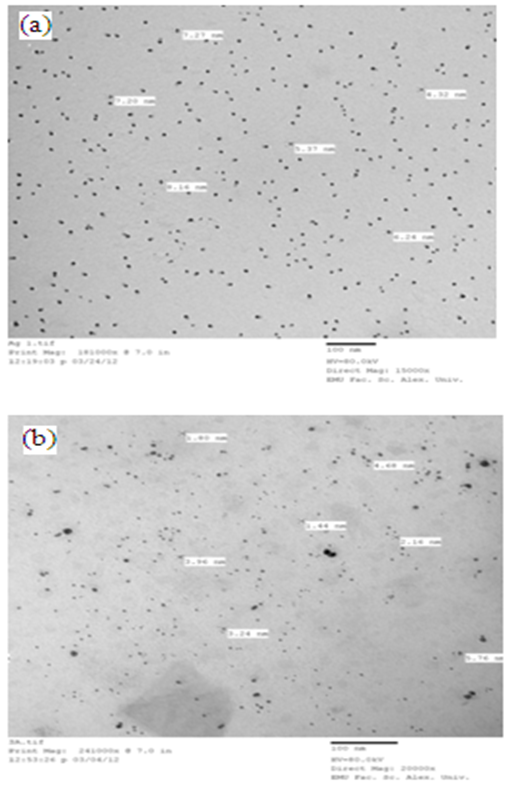
3.1.1. Qualitative Assessment by TEM
- TEM visualization allows the measurement of the size and shape of the synthesized nanoparticles. The TEM images of biologically synthesized Ag-NPs using ginger extract (figure 3) confirm that the obtained Ag-NPs are spherical in shape with diameters ranging from 1.44 to 5.76 nm and an average size of 3.1 nm.
3.1.2. Confirmation of Ag-NPs by XRD
- XRD is a very important technique that is commonly used to analyze the characteristics and structural details of nanomaterials. The XRD patterns are obtained by measuring the angles at which an X-ray beam is diffracted by the crystalline phases in the specimen. The XRD pattern of green synthesized Ag-NPs is shown in figure 4, where four prominent peaks at 38° (2θ); 44o (2θ); 64o (2θ) and 77o (2θ) indicated the presence of (111), (200), (220) and (311) sets of lattice planes, and accordingly could be indexed as face-centered–cubic structures of Ag-NPs. Hence, from the XRD pattern it is clear that Ag-NPs formed using ginger rhizome broth were essentially crystalline in nature. The average crystalline particle size for green synthesized Ag-NPs determined by Scherre’s formula using the half width of the most intense XRD peaks was 4.17±1.12 nm. It can be noted that the size of the Ag-NPs obtained from TEM is in agreement with the size obtained from the XRD measurements.
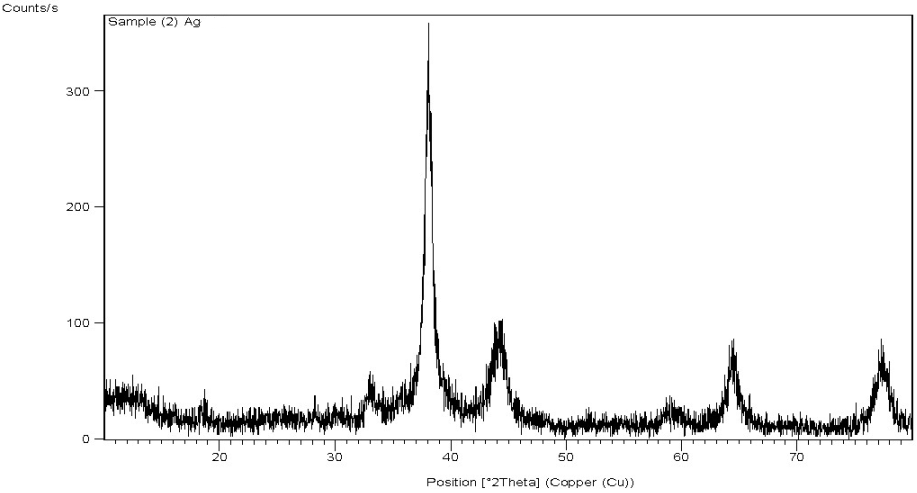 | Figure 4. XRD spectrum of green synthesized of Ag-NPs using Zingiber officinale extract |
3.1.3. Mechanism of Formation of Ag-NPs by FTIR
- FTIR measurements were performed to identify the potential biomolecules in the ginger rhizome responsible for the reduction and then provision of stability to the bio-reduced Ag-NPs. Figure 5 displays the FTIR spectra of Ag-NPs; where the peak was centered at 1384 cm-1 which indicated the presence of NO3- in the residual solution. The band at 3419 cm-1 corresponds to O-H stretching H-bonded alcohols and phenols. The peak at 2923 cm-1 corresponds to O-H stretch carboxylic acids. The assignment at 1648 cm-1 corresponds to N-H bend primary amines. The peak at 1376 cm-1 corresponds to C-N stretching of aromatic amine group and the bands observed at 1163, 1113, 1059 cm-1corresponds to C-N stretching alcohols, carboxylic acids, ethers and esters. The various functional groups mentioned here are mainly derived from heterocyclic compounds that are the water soluble components of ginger rhizome. So it can be assumed that different water soluble heterocyclic compounds such as alkanoids and flavonoids functioned as the capping ligands for the synthesis of Ag-NPs [18].
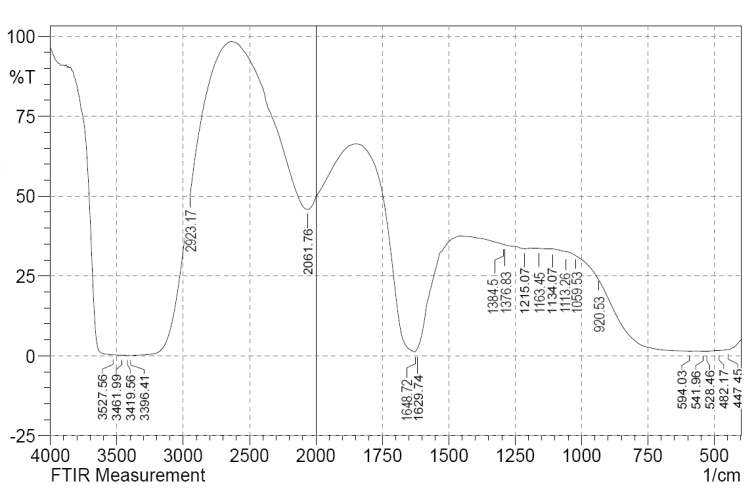 | Figure 5. FTIR spectrum of the Ag-NPs synthesized by the reduction of AgNO3 using Zingiber officinale extract |
3.2. Antibacterial Activity of Ag-NPs
- Antibacterial effect of Ag-NPs was studied against two different pathogenic bacteria; S.aureus and E.coli strains.
3.2.1. Antibacterial Assay of Ag-NPs
- The effect of Ag-NPs on bacteria was performed using disc diffusion method. As shown in figure 6; larger clear inhibition zones surrounded the discs impregnated with Ag-NPs (50µL, 3mM) as compared to the standard control antibiotics used.
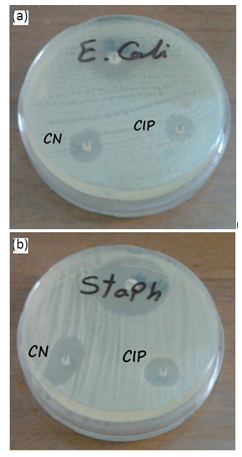 | Figure 6. Antibacterial activity of 3mM Ag-NPs solution green synthesized by ginger extract (upper disc) and Gentamycin (CN), and Ciprofloxacin(CIP) used against, E. coli(a)and S. aureus (b) |
3.2.2. Estimation of CFU
- Figure 7 shows the plot of number of bacterial colonies grown on nutrient agar plates as a function of concentration of Ag-NPs. The colony count was significantly reduced with the increasing concentrations of Ag-NPs.
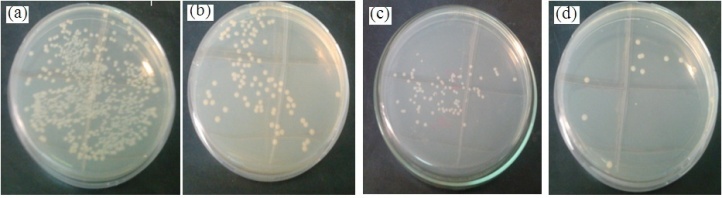 | Figure 7. Digital photographs of E. coli colonies grown on nutrient agar plate as a function of silver nanoparticles concentration. a) Control, b) 20 µg/ml, c) 40 µg/ml and d) 60 µg/ml |
4. Conclusions
- Plants or their extracts can be efficiently used in the synthesis of Ag-NPs as a greener route. Control over the shape and size of nanoparticles seems to be very easy with the use of plants. In the present study we found that ginger rhizome may be be good source for the synthesis of Ag-NPs. This green approach for synthesis of Ag-NPs has many advantages such including the ease with which the process can be scaled up and its economic viability.The use of such eco-friendly nanoparticles as a bactericidal agent in medical and electronic applications, renders this method potentially exciting for a large-scale synthesis of other inorganic materials (nanomaterials).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML