-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(1): 7-13
doi:10.5923/j.nn.20150501.02
Thermal and Mechanical Properties of Chitosan / Mimosa Tenuiflora / Multiwalled Carbon Nanotubes Composite Developed by Thermally Induced Phase Separation
S. A. Martel-Estrada1, Olivas-Armendáriz2, L. Peón-Prieto1, P. Urquizo-Monrreal1, N. Hernández-Osuna1, C. A. Martínez-Pérez2, J. L. Hernández-Arellano1, E. Santos-Rodríguez3
1Instituto de Arquitectura, Diseño y Arte, Universidad Autónoma de Ciudad Juárez, Chih., México
2Instituto de Ingeniería y Tecnología, Universidad Autónoma de Ciudad Juárez, Chih., México
3UNACH/MCTP, Ciudad Universitaria Carretera Emiliano Zapata Km. 4, Chiapas, México
Correspondence to: E. Santos-Rodríguez, UNACH/MCTP, Ciudad Universitaria Carretera Emiliano Zapata Km. 4, Chiapas, México.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The inclusion of multiwall carbon nanotubes (MWCNTs) into polymeric matrices is expected to improve their mechanical and thermal properties. In this work, biodegradable composites of chitosan/mimosa tenuiflora/multiwalled carbon nanotubes have been prepared through thermally induced phase separation method, in order to evaluate their thermal and mechanical properties for applications in tissue engineering. Different compositions were analyzed. Variation in porous morphology of the scaffold was studied using a field emission scanning electron microscopy (SEM). The morphology of the composites developed indicates a highly interconnected porous structure with a diameter from 1-100 µm. Presence of MWCNT improves the thermal stability and mechanical properties of the chitosan/mimosa tenuiflora scaffold. The results from IR, TGA, DSC and mechanical properties suggest that there is a chemical interaction between the constituents of the composite.
Keywords: Chitosan, MWCNT, Mimosa Tenuiflora, TGA, DSC, SEM
Cite this paper: S. A. Martel-Estrada, Olivas-Armendáriz, L. Peón-Prieto, P. Urquizo-Monrreal, N. Hernández-Osuna, C. A. Martínez-Pérez, J. L. Hernández-Arellano, E. Santos-Rodríguez, Thermal and Mechanical Properties of Chitosan / Mimosa Tenuiflora / Multiwalled Carbon Nanotubes Composite Developed by Thermally Induced Phase Separation, Nanoscience and Nanotechnology, Vol. 5 No. 1, 2015, pp. 7-13. doi: 10.5923/j.nn.20150501.02.
Article Outline
1. Introduction
- Biological systems perform complex macroscale tasks. The first level of cellular organization is composed of macromolecules with lengths at the nanoscale. Nanoscale have demonstrated regulatory effects on cell behavior, including adhesion, migration, proliferation, signaling, genetic expression and stem cell fate. For these reasons, biomaterials are being designed with nanoscale details to improve cellular behavior [1]. Chitosan is one of the most popular natural polymers used in tissue engineering applications [2]. Chitosan can be obtained from chitin by deacetylation [2, 3]. It is a polysaccharide composed of glucosamine and N-acetyl glucosamine linked with a β1-4 glucosidic linkage [2]. It is biocompatible and biodegradable [2]. Nevertheless, some of its properties, such as bioactivity [2, 4], mechanical properties, etc., need to be improved. Chitosan can form porous structure by thermally induced phase separation [4, 5].Carbon nanotubes (CNTs) are graphitic tubular structures with extraordinary physical, electrical and chemical properties [1, 6]. They have an excellent potential to reinforce polymeric matrices because of their small dimensions, specially high aspect ratio, strength and stiffness [7]. They can improve mechanical properties and biocompatibility, modulate cell shape, regulate stem cell differentiation and improve proliferation [1, 8]. There are two subsets of CNT: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). Mimosa Tenuiflora (Willd) Poiret is a species of the Mimosoideae, a subfamily of the Fabaceae botanical family in the south of Mexico, Venezuela and Brazil [4]. Mimosa Tenuiflora contains alkaloids, mainly N-N- dimethyltryptamine, serotonin, triperpenoid glycosides, and steroidal saponins [4, 9].Previously, an effort has been devoted to evaluate the potential use of chitosan/mimosa tenuiflora composite as a bioactive material [4]. Although this blend showed good bioactivity, the mechanical properties must be improved. So, in this work, biodegradable composites of chitosan/ mimosa tenuiflora/multiwalled carbon nanotubes have been prepared through thermally induced phase separation method, in order to evaluate their thermal and mechanical properties for application in tissue engineering.
2. Experimental
2.1. Materials
- Chitosan (75-85% deacetylated) with molecular weight of 310,000-376,000 Da (75 -85% deacetylated) was purchased from Sigma-Aldrich (United States). The bark of M. Tenuiflorawas obtained directly from the Jiquipilas Valley (Chiapas, Mexico). It was ground to powder using a grinder. without any preparation. Glacial acetic acid (Mallinckrodt, United States) was used as a solvent for the blends. The MWCNT were fabricated in our laboratory by spray pyrolysis method according with a previous research [10].
2.2. Preparation of Nanocomposite
- Chitosan/M. Tenuiflora/Multiwalled carbon nanotubes (MWCNT) composite was prepared using the thermally induced phase separation (TIPS) technique. It was prepared 80/20 Ch/M. Tenuiflora composites to which it was added 1% and 2% of MWCNT. First, measured percentage of the purified MWCNT was dispersed in 1% (v/v) aqueous glacial acetic acid by sonication for 5 min at room temperature. Then, Chitosan and M. Tenuiflora were added to the MWCNT solution and mixed together at an 80/20 ratio. Finally, the composite was frozen at -20℃ for 4 h and then the solvent was extracted by a freeze-drying in Labconco Free Zone 2.5 for 48 hours.The composites were neutralized by immersion in ethanol at -20°C during 12 hours. It was prepared an 80% ethanol aqueous solution, to which it was added 0.5(w%/w%) of NaOH. The composites were immersed into the NaOH-CH3CH2OH solution at the same temperature during 12 hours more. Then, the samples were washed and rinsed with distilled water.
2.3. Characterization
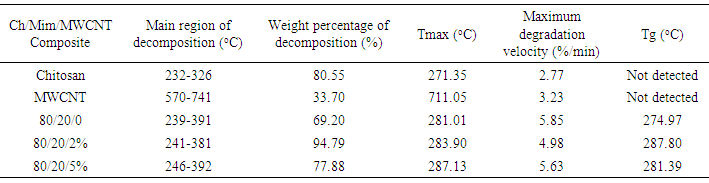
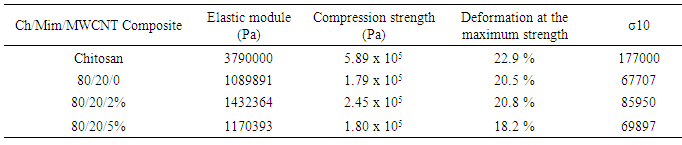
- Chemical characterization of the composites was made using a FTIR-ATR spectrometer (Nicolet 6700). All spectra were recorded at 100 scans and 16 cm−1 resolution. X-ray diffraction patterns of the samples were obtained at 2θvalues between 5° and 80° with a step size of 0.02° using an X-ray diffractometer using (PANanalyticalX’Pert PRO) operated in continuous mode. The morphology of the composite was characterized using a Field Emission Scanning Electron Microscope (JEOL JSM-7000F).Thermogravimetric analysis (TGA) and Differential Scanning Calorimetry (DSC) of the composite was carried out using TG-DSC analyzer (SDT Q600 from TA Instruments, United States). Dry samples were heated from 30℃ to 750℃at 10℃/min under nitrogen atmosphere with a flow rate of 80 mL/min. The first derivative of the mass-change with respect to time (DTG) was calculated and plotted as a function of the temperature. The average pore size and pore size distribution of the composite was measured from the SEM micrographs. For this purpose, it was used the Scandium Universal SEM Imaging Platform software (Soft Imaging System) in the original magnification. Three different cross-sections of each scaffold were used to estimate the pore size and at least 120 pores were measured to estimate the distribution. Finally, RSA III (Rheometrics Analyzes System) TA was used to elaborate a stress-strain analysis under a compression clamp in a frequency of 6.2832 rad/s and in a temperature 37℃, for all samples. Disks of approximately 5 mm in thickness and 15 mm in diameter were used for this test.
3. Results and Discussion
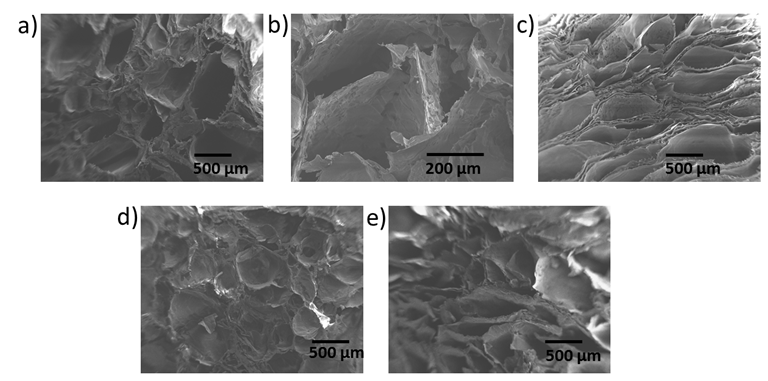
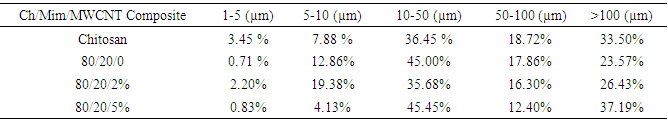
- The microstructural properties of the scaffold play an important role to tissue repair, including pore diameter that influence cell attachment, proliferation and migration [11]. Pore size desired for optimal cellular activity and viability to interact with proteins and bioactivity and cellular development is 20-120 μm [5, 12]. The morphology of the composites as shown in Figure 1 indicates a highly interconnected porous structure. The porous structure consists of pores ranging in diameter from 1-100 µm. Table 1 shows those ranges of poresizes for the composites developed in this study.
|
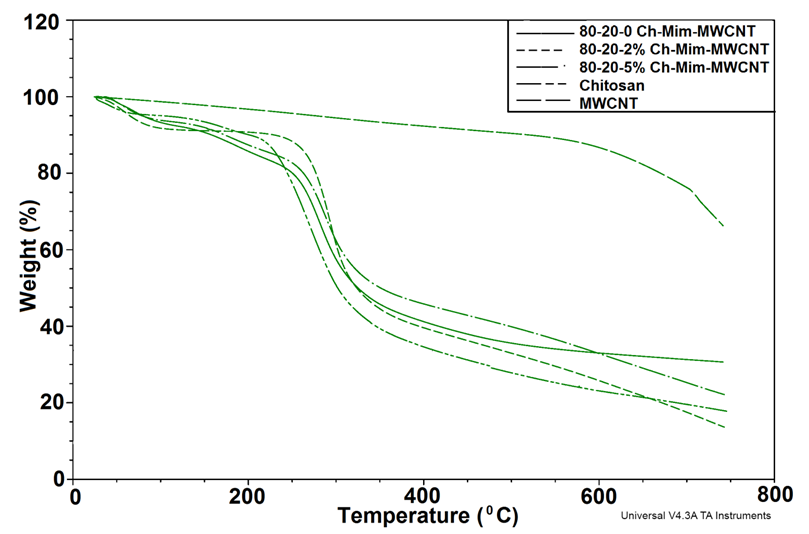 | Figure 2. TGA curves showing the thermal degradation of the composites |
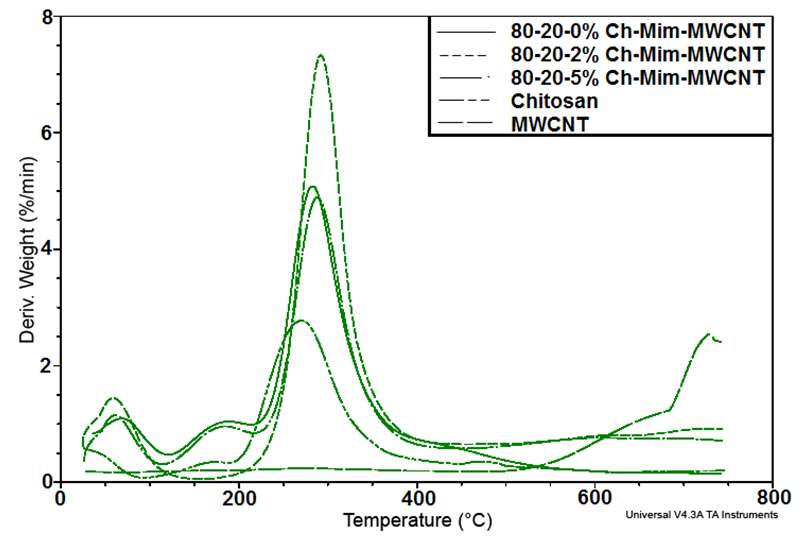 | Figure 3. DTG curves showing the thermal degradation of the composites |
|
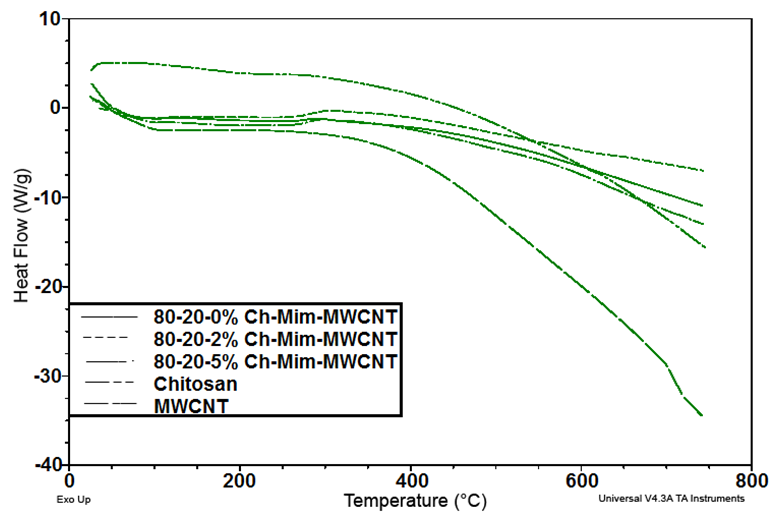 | Figure 4. DSC curves of the composites |
|
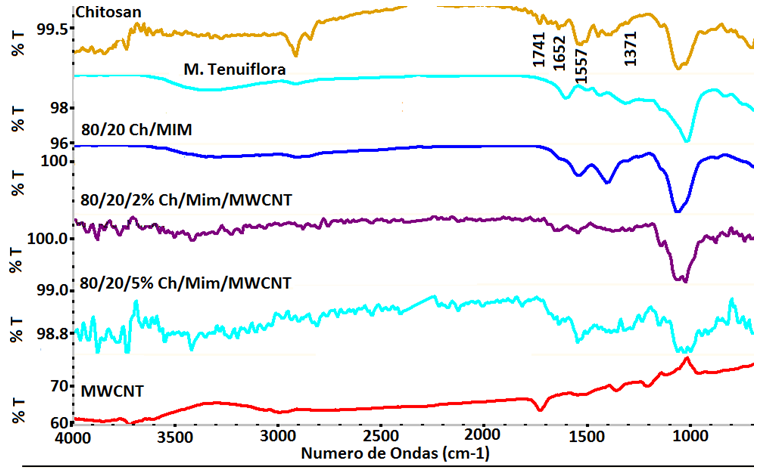 | Figure 5. FTIR spectra of Chitosan/M. Tenuiflora/MWCNT composites |
4. Summary
- The present study provides more insight into thermophysical properties of Chitosan/Mimosa Tenuiflora/ MWCNT scaffolds. The composite specimens were successfully fabricated by thermally induced phase separation (TIPS) method. The microstructural properties of the scaffold play an important role to tissue repair. The morphology of the composites developed, indicates a highly interconnected porous structure with a diameter from 1-100 µm. So, according with the morphology obtained, all the composites developed in this study could be suitable for bone engineering. Presence of MWCNT was found to improve the thermal stability and mechanical properties of the chitosan/mimosa tenuiflora scaffold.Thermal decomposition of the composites was greatly improved with the inclusion of MWCNT. The thermal degradation of composites fabricated started at the higher temperature compared to chitosan. The obtained results from IR, TGA, DSC and mechanical properties suggest a chemical interaction between the polymers. It is believed that an interaction occurred between the amino group (mainly in chitosan) and carboxyl groups in MimosaTenuiflora or hydroxyl (mainly in chitosan) and carboxyl groups in the blends. Also, these events suggested that it was formed hydrogen bond interaction with MWCNT. The results show improved mechanical and thermal properties of the composite compared to Chitosan/Mimosa Tenuiflora composites, which can provide better stability of the scaffold. However, biocompatibility studies are required to confirm the suitability of the composites for bone engineering.
ACKNOWLEDGEMENTS
- The authors acknowledge the financial support of the Mexican Public Education Secretary and the Mexican National Council for Science and Technology (CONACyT) through Projects SEP-CONACyT CB 2012-01-180909 and PROMEP/103.5/13/2347. Also, the authors appreciate the support of Mr. Armando Cardona-Torres during the edition of this document.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML